Q1) i- Estimate the diffusivity of carbon oxide (CO2) in air at 330 K and 1.2 bar applying the following formula and the data given in Table Q1. (0.98 3.03 (10-3) T1.5 MO.5 AB P MO.5 oAB Np DAB 'AB where; DAB = diffusion coefficient, cm?/sec MA , MB = molecular weights of A and B, respectively T= temperature, °K P= Pressure, bar O AB= collision diameter, angstrom Np = diffusion collision integral, dimensionless Given the following: -1 МАВ 3 2 \MA MB O AB = 0.5(0A +0B) 0.5 EA EB к к E AB K II
Q1) i- Estimate the diffusivity of carbon oxide (CO2) in air at 330 K and 1.2 bar applying the following formula and the data given in Table Q1. (0.98 3.03 (10-3) T1.5 MO.5 AB P MO.5 oAB Np DAB 'AB where; DAB = diffusion coefficient, cm?/sec MA , MB = molecular weights of A and B, respectively T= temperature, °K P= Pressure, bar O AB= collision diameter, angstrom Np = diffusion collision integral, dimensionless Given the following: -1 МАВ 3 2 \MA MB O AB = 0.5(0A +0B) 0.5 EA EB к к E AB K II
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
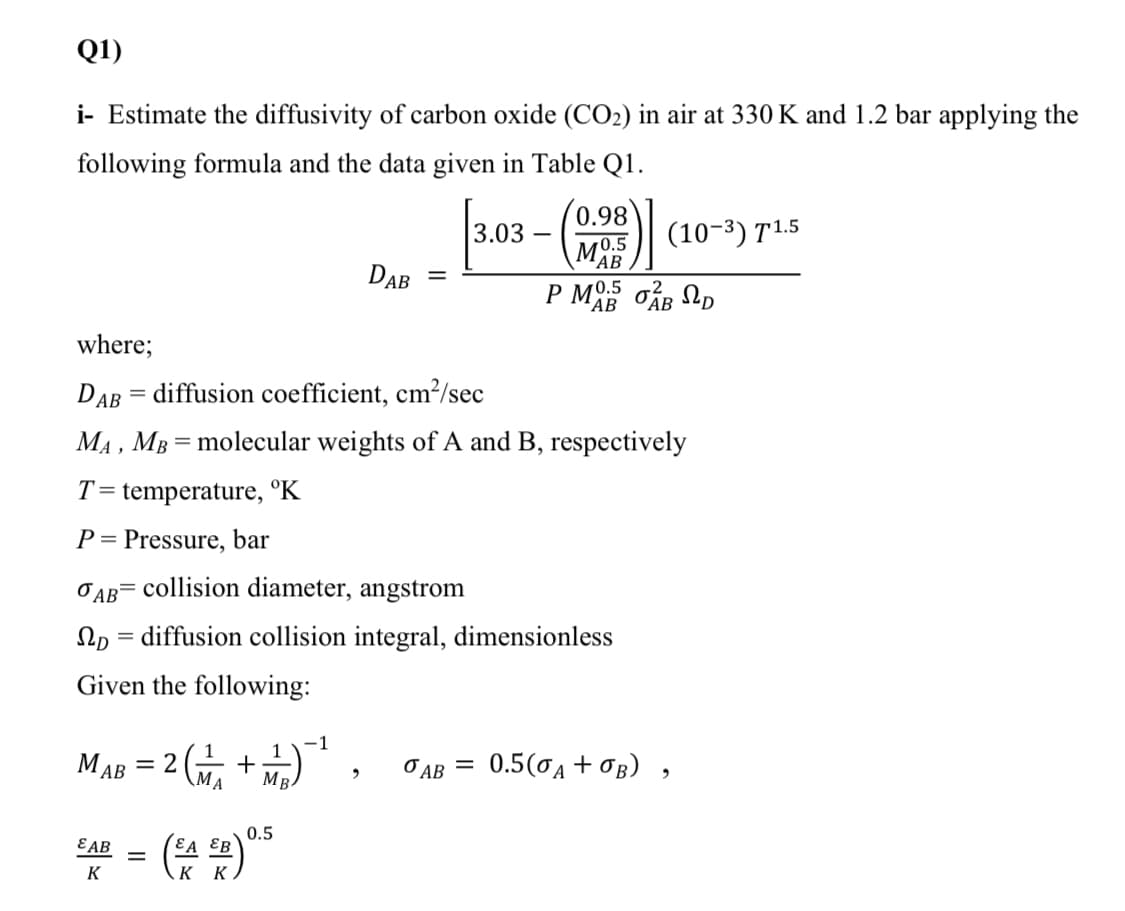
Transcribed Image Text:Q1)
i- Estimate the diffusivity of carbon oxide (CO2) in air at 330 K and 1.2 bar applying the
following formula and the data given in Table Q1.
(0.98
3.03
(10-3) T1.5
MO.5
'AB
DAB
АВ
where;
DAB = diffusion coefficient, cm²/sec
MA , MB = molecular weights of A and B, respectively
T= temperature, °K
P = Pressure, bar
O AB= collision diameter, angstrom
Np = diffusion collision integral, dimensionless
Given the following:
–1
1
MAB = 2 +)*, JAB = 0.5(0A + Og) ,
МАВ
0.5(0A + OB)
%3D
MB.
O AB =
0.5
EA ƐB
εΑΒ
K
K K
„G)
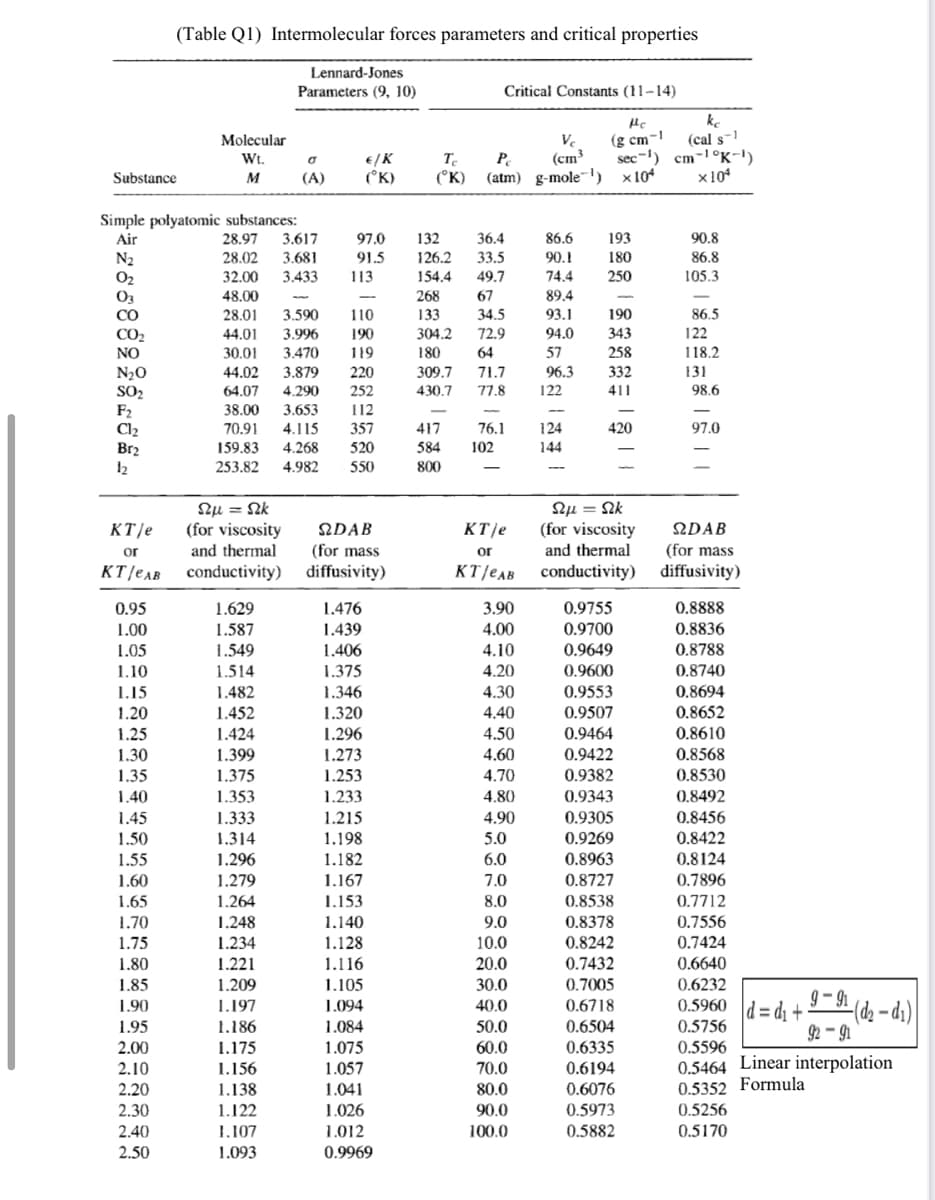
Transcribed Image Text:(Table Q1) Intermolecular forces parameters and critical properties
Lennard-Jones
Parameters (9, 10)
Critical Constants (11-14)
(g cm-
sec-1)
ke
(cal s-1
cm-1°K-')
x10
Molecular
(cm³
g-mole¬')
Wt.
€/K
((K)
Te
((K)
Pe
Substance
M
(A)
(atm)
x 10
Simple połyatomic substances:
3.617
3.681
Air
28.97
97.0
86.6
193
90.8
132
126.2
36.4
33.5
86.8
N2
O2
28.02
91.5
90.1
180
32.00
3.433
113
154.4
49.7
74.4
250
105.3
89.4
93.1
48.00
268
67
CO
28.01
3.590
110
133
34.5
190
86.5
CO2
3.996
190
304.2
72.9
94.0
343
122
44.01
30.01
NO
3.470
119
180
64
57
258
118.2
3.879
220
309.7
71.7
96.3
332
131
N20
SO2
F2
Cl2
Br2
12
44.02
64.07
4.290
252
430.7
77.8
122
411
98.6
38.00
3.653
112
70.91
4.115
357
417
76.1
124
420
97.0
159.83
4.268
520
584
102
144
253.82
4.982
550
800
Nµ = Nk
(for viscosity
and thermal
ΩμΩk
(for viscosity
KT/e
NDAB
KT/e
NDAB
(for mass
(for mass
conductivity) diffusivity)
or
or
and thermal
KT/eAB
KT/eAB
conductivity) diffusivity)
0.9755
0.9700
1.629
3.90
4.00
0.8888
0.8836
0.8788
0.8740
0.8694
0.95
1.476
1.587
1.549
1.439
1.406
1.00
1.05
4.10
0.9649
1.10
1.514
1.375
4.20
0.9600
1.482
1.452
1.15
1.346
4.30
0.9553
1.20
1.320
4.40
0.9507
0.8652
0.9464
0.9422
1.25
1.424
1.296
4.50
0.8610
1.273
1.253
1.30
1.399
4.60
0.8568
1.35
1.375
4.70
0.9382
0.8530
1.40
1.353
1.233
4.80
0.9343
0.8492
1.215
1.198
1.182
0.9305
0.9269
1.45
1.333
4.90
0.8456
1.50
1.314
5.0
0.8422
1.55
1.296
6.0
0.8963
0.8124
0.8727
0.8538
1.60
1.279
1.167
7.0
0.7896
1.264
1.248
1.234
1.221
1.65
1.153
8.0
0.7712
1.140
1.128
1.70
9.0
0.8378
0.7556
1.75
10.0
0.8242
0.7424
1.80
1.116
20.0
0.7432
0.6640
1.85
1.209
1.105
30.0
0.7005
0.6232
9 – 9.
-(d2 – d1)
0.5960 d= d +
92 – 91
1.90
1.197
1.094
40.0
0.6718
1.084
1.075
1.95
1.186
50.0
0.6504
0.5756
60.0
0.5596
0.5464 Linear interpolation
0.5352 Formula
2.00
1.175
0.6335
2.10
1.156
1.057
70.0
0.6194
1.041
1.026
80.0
90.0
2.20
1.138
0.6076
0.5973
0.5882
2.30
1.122
0.5256
2.40
1.107
1.012
100.0
0.5170
2.50
1.093
0.9969
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The