Q4 Ethylene is a valuable feedstock for many chemical processes. In future years, when petroleum is not as readily available, ethylene may be produced by dehydration of ethanol: C₂H₂OH=C₂H₂+H₂O Note that ethanol may be readily obtained by fermentation of biomass. All reactants and products can be considered ideal gases. The feed stream is 1mol ethanol and 1mol N₂, stand states is at 1 bar Species 1 (C₂H5OH) 2 (C₂H4) 3 (H₂O) 4 (N₂) Sum a) c) ΔΗ°1,298 kJ/mol -1 -234.95 Vi 1 Kat= 1 -241.835 ΝΑ 52.51 ΝΑ 0 ΝΑ AG°f,298 kJ/mol -167.73 68.43 -228.614 0 ΝΑ n} \n{ = n{+v₁5 1 0 0 1 ΝΑ Yi Fill in the blanks in the table above, use as the extent of reaction. y; is the molar fraction of species i in the gas phase. b) Calculate the equilibrium constant Ka for this reaction at 298K using standard state Gibbs energy of reaction AG 298K Kar= Calculate the equilibrium constant Ka for this reaction at 400 K using the short cut Van't Hoff equation. d) . Write down an equation that allows you to calculate if the reaction happens at 400 K and 1 bar. You don't need to solve for . e) If the system remains at 400 K, but its pressure increases to 2 bar, does increase or decrease compared to when system pressure is 1 bar?
Q4 Ethylene is a valuable feedstock for many chemical processes. In future years, when petroleum is not as readily available, ethylene may be produced by dehydration of ethanol: C₂H₂OH=C₂H₂+H₂O Note that ethanol may be readily obtained by fermentation of biomass. All reactants and products can be considered ideal gases. The feed stream is 1mol ethanol and 1mol N₂, stand states is at 1 bar Species 1 (C₂H5OH) 2 (C₂H4) 3 (H₂O) 4 (N₂) Sum a) c) ΔΗ°1,298 kJ/mol -1 -234.95 Vi 1 Kat= 1 -241.835 ΝΑ 52.51 ΝΑ 0 ΝΑ AG°f,298 kJ/mol -167.73 68.43 -228.614 0 ΝΑ n} \n{ = n{+v₁5 1 0 0 1 ΝΑ Yi Fill in the blanks in the table above, use as the extent of reaction. y; is the molar fraction of species i in the gas phase. b) Calculate the equilibrium constant Ka for this reaction at 298K using standard state Gibbs energy of reaction AG 298K Kar= Calculate the equilibrium constant Ka for this reaction at 400 K using the short cut Van't Hoff equation. d) . Write down an equation that allows you to calculate if the reaction happens at 400 K and 1 bar. You don't need to solve for . e) If the system remains at 400 K, but its pressure increases to 2 bar, does increase or decrease compared to when system pressure is 1 bar?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
Need , a,b and d part. As per guidelines.
Please solve it correctly!!! Othwrwise skip
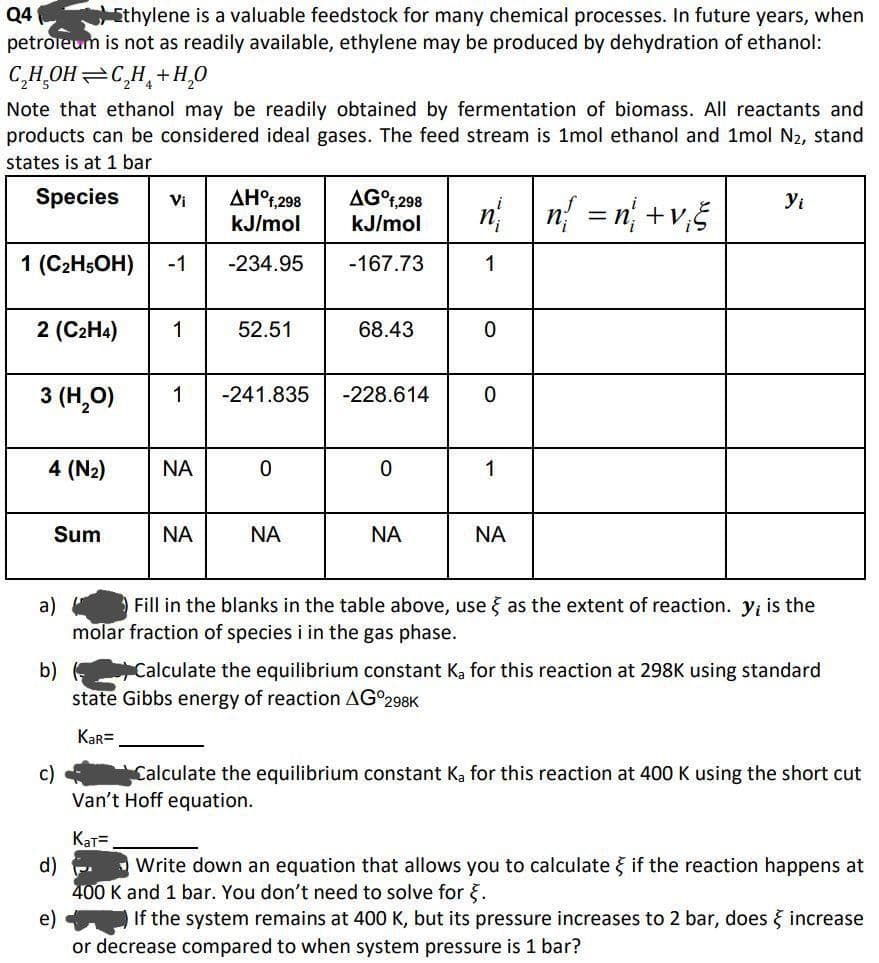
Transcribed Image Text:Q4 Ethylene is a valuable feedstock for many chemical processes. In future years, when
petroleum is not as readily available, ethylene may be produced by dehydration of ethanol:
C₂H₂OH C₂H+H₂O
Note that ethanol may be readily obtained by fermentation of biomass. All reactants and
products can be considered ideal gases. The feed stream is 1mol ethanol and 1mol N₂, stand
states is at 1 bar
Species
1 (C₂H5OH)
2 (C₂H4)
3 (H₂O)
4 (N₂)
Sum
a)
Vi
1
Kat=
ΝΑ
AHᵒf,298
kJ/mol
-234.95
1 -241.835
ΝΑ
52.51
0
ΝΑ
AG°f,298
kJ/mol
-167.73
68.43
-228.614
0
ΝΑ
nn = n+v₁5
1
0
0
1
ΝΑ
Yi
Fill in the blanks in the table above, use as the extent of reaction. y; is the
molar fraction of species i in the gas phase.
b) (
Calculate the equilibrium constant Ka for this reaction at 298K using standard
state Gibbs energy of reaction AG°298K
KaR=
c)
Calculate the equilibrium constant Ka for this reaction at 400 K using the short cut
Van't Hoff equation.
d) -
Write down an equation that allows you to calculate } if the reaction happens at
400 K and 1 bar. You don't need to solve for .
e)
If the system remains at 400 K, but its pressure increases to 2 bar, does & increase
or decrease compared to when system pressure is 1 bar?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The