QI/A 0.1 mole% caustic soda (NaOH) solution is to be (concentrated in a continuous evaporator. The solution enters the unit at 25 °C at a rate of 150 mol/min and is concentrated to 5 mole% at 50 °C.1061mol/min of hot dry air at 200 °C and 1 atm is bushed through the evaporator and leaves form the top exit at 50 °C andl atm. The saturated water vapor leaves the evaporator at 50 °C and 1 atm. Calculate the rate at which heat must be transferred to or from the unit. Also, if the air flowrate is decreased to half how that would affect the transferred heat? Show that effect in percentage. 1061 CANaOn 4.184 J/g C Enthalpy of statured steam at 50 C-44.81 KJ/mol He Dry air dry air mol/min
QI/A 0.1 mole% caustic soda (NaOH) solution is to be (concentrated in a continuous evaporator. The solution enters the unit at 25 °C at a rate of 150 mol/min and is concentrated to 5 mole% at 50 °C.1061mol/min of hot dry air at 200 °C and 1 atm is bushed through the evaporator and leaves form the top exit at 50 °C andl atm. The saturated water vapor leaves the evaporator at 50 °C and 1 atm. Calculate the rate at which heat must be transferred to or from the unit. Also, if the air flowrate is decreased to half how that would affect the transferred heat? Show that effect in percentage. 1061 CANaOn 4.184 J/g C Enthalpy of statured steam at 50 C-44.81 KJ/mol He Dry air dry air mol/min
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Hi I need step by step handwritten answer please
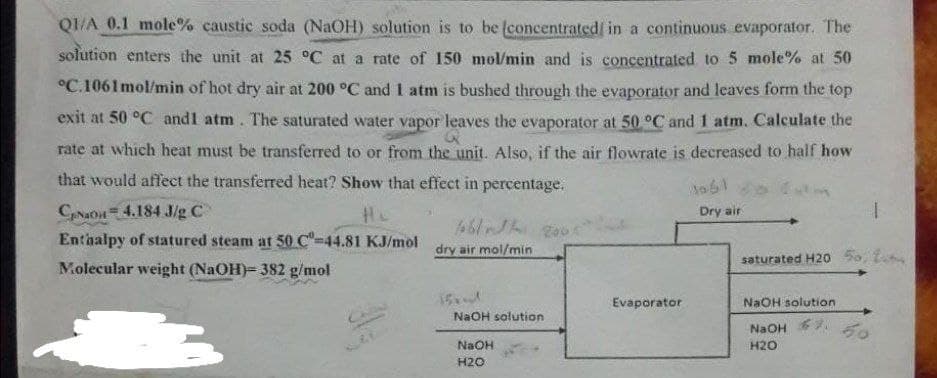
Transcribed Image Text:QI/A 0.1 mole% caustic soda (NaOH) solution is to be (concentrated/ in a continuous evaporator. The
solution enters the unit at 25 °C at a rate of 150 mol/min and is concentrated to 5 mole% at 50
°C.1061mol/min of hot dry air at 200 °C and 1 atm is bushed through the evaporator and leaves form the top
exit at 50 °C andl atm. The saturated water vapor leaves the evaporator at 50 °C and 1 atm. Calculate the
rate at which heat must be transferred to or from the unit. Also, if the air flowrate is decreased to half how
that would affect the transferred heat? Show that effect in percentage.
CNaOn 4.184 J/g C
Enthalpy of statured steam at 50 C"-44.81 KJ/mol
Dry air
dry air mol/min
saturated H20 5o, t
Molecular weight (NaOH)= 382 g/mol
15
Evaporator
NaOH solution
NaOH solution
NaOH
50
NaOH
H20
H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The