QUESTION 4 In the methane molecule. CH4. each hydrogen atom is at the corner of a regular tetrahedron with the carbon atom at the center. It ane of the C-H is in the direction of A=7+7+R and an adjacent CH bond is at the direction B-7-7-R. results to an angular bond of approximately 109° for a static frozen molecule. However, the molecule we can encounter everyday continuously vibrates and interact with the surrounding causing its bond vector to vary slightly. According to a new spectroscopy analysis. the adjacent bond vectors was found to be A- 1.09i + 0.92) + 1.02k 8- 0.88i + 0.87j+ 1.06k What is the angle tin degrees) betwoen the bonds based on this new data? Note: Only 19% of error is permitted for the correct answer.
QUESTION 4 In the methane molecule. CH4. each hydrogen atom is at the corner of a regular tetrahedron with the carbon atom at the center. It ane of the C-H is in the direction of A=7+7+R and an adjacent CH bond is at the direction B-7-7-R. results to an angular bond of approximately 109° for a static frozen molecule. However, the molecule we can encounter everyday continuously vibrates and interact with the surrounding causing its bond vector to vary slightly. According to a new spectroscopy analysis. the adjacent bond vectors was found to be A- 1.09i + 0.92) + 1.02k 8- 0.88i + 0.87j+ 1.06k What is the angle tin degrees) betwoen the bonds based on this new data? Note: Only 19% of error is permitted for the correct answer.
Trigonometry (MindTap Course List)
8th Edition
ISBN:9781305652224
Author:Charles P. McKeague, Mark D. Turner
Publisher:Charles P. McKeague, Mark D. Turner
Chapter3: Radian Measure
Section3.5: Velocities
Problem 60PS: Gear Trains Figure 8 shows a single-stage gear train. Gear trains are used in many products, such as...
Related questions
Question
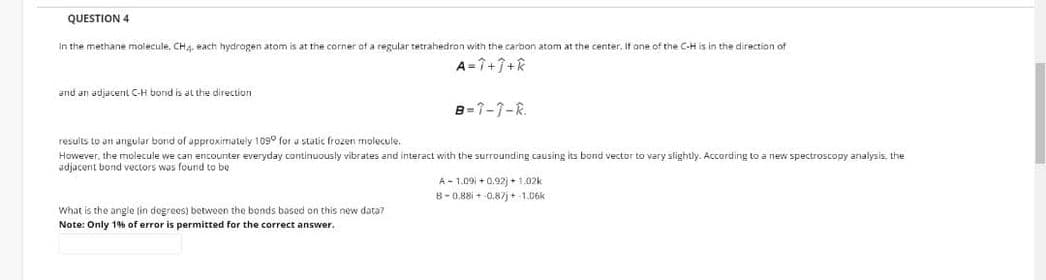
Transcribed Image Text:QUESTION 4
In the methane molecule. CH4. each hydrogen atom is at the corner of a regular tetrahedron with the carbon atom at the center, It one of the C-H is in the direction of
A=7+7+k
and an adjacent C-H bond is at the direction
B=1-1-R.
results to an angular bond of approximately 109° for a static frozen molecule.
However, the molecule we can encounter everyday continuously vibrates and interact with the surrounding causing its bond vector to vary slightly. Accerding to a new spectroscopy analysis, the
adjacent bond vectors was found to be
A - 1.09 + 0,92) + 1.02k
8-0.88i + 0.87j +-1.06k
What is the ange tin degrees) between the bonds based on this new data?
Note: Only 1% of error is permitted for the correct answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, calculus and related others by exploring similar questions and additional content below.Recommended textbooks for you

Trigonometry (MindTap Course List)
Trigonometry
ISBN:
9781305652224
Author:
Charles P. McKeague, Mark D. Turner
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Trigonometry (MindTap Course List)
Trigonometry
ISBN:
9781305652224
Author:
Charles P. McKeague, Mark D. Turner
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage