Reacting with water in an acidic solution at 35°C, sucrose (C H,,O ) decomposes into glucose (C,H,06) and fructose (C,H,0,) according to the law of uninhibited decay. An initial amount of 0.7 mol of sucrose decomposes to 0.63 mol in 15 min. How many moles A of sucrose will remain after 5 h, and how many hours t will it take before only 0.59 mol of sucrose remain? (Provide each answer correct to two decimal places.) A = mol h
Reacting with water in an acidic solution at 35°C, sucrose (C H,,O ) decomposes into glucose (C,H,06) and fructose (C,H,0,) according to the law of uninhibited decay. An initial amount of 0.7 mol of sucrose decomposes to 0.63 mol in 15 min. How many moles A of sucrose will remain after 5 h, and how many hours t will it take before only 0.59 mol of sucrose remain? (Provide each answer correct to two decimal places.) A = mol h
College Algebra
7th Edition
ISBN:9781305115545
Author:James Stewart, Lothar Redlin, Saleem Watson
Publisher:James Stewart, Lothar Redlin, Saleem Watson
Chapter4: Exponential And Logarithmic Functions
Section: Chapter Questions
Problem 99E: Radioactive decay Uranium-234 has a half-life of 2.7x105 Years. (a)Find the amount remaining from a...
Related questions
Question
Please help me solve. Thank you!
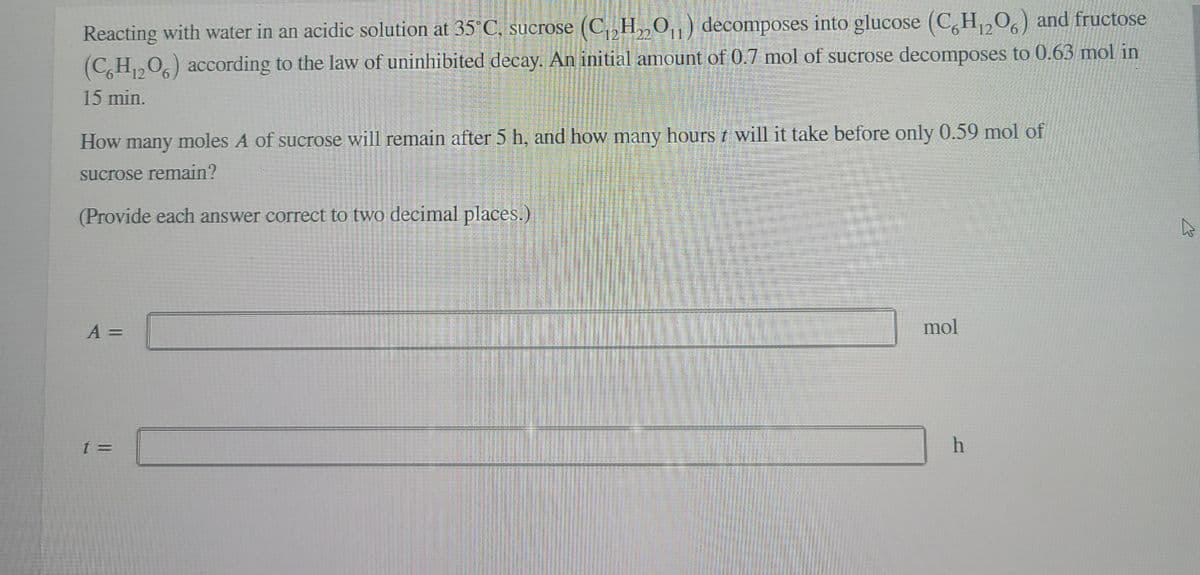
Transcribed Image Text:Reacting with water in an acidic solution at 35°C, sucrose (C, H„0,) decomposes into glucose (C,H,0) and fructose
(C,H,0,) according to the law of uninhibited decay. An initial amount of 0.7 mol of sucrose decomposes to 0.63 mol in
12
15 min.
How many moles A of sucrose will remain after 5 h, and how many hours t will it take before only 0.59 mol of
sucrose remain?
(Provide each answer correct to two decimal places.)
A =
mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, calculus and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Algebra
Algebra
ISBN:
9781305115545
Author:
James Stewart, Lothar Redlin, Saleem Watson
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

College Algebra
Algebra
ISBN:
9781305115545
Author:
James Stewart, Lothar Redlin, Saleem Watson
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage