See graph. There is 2 mol of an ideal monatomic gas. The following two point, 4, 2 are given below, 4: P4 = 2atm, T4 = 360K. 2: V2 = 3V4 and P2 = 2P3. 1- 2 Isothermal process. 2 3 → 4 Isothermal process. 3 V The cycle proceeds from 4 →1→ 2 – 3 → 4. Determine the total amount of work done by the gas and the heat supplied to the gas along each portion of the cycle.
See graph. There is 2 mol of an ideal monatomic gas. The following two point, 4, 2 are given below, 4: P4 = 2atm, T4 = 360K. 2: V2 = 3V4 and P2 = 2P3. 1- 2 Isothermal process. 2 3 → 4 Isothermal process. 3 V The cycle proceeds from 4 →1→ 2 – 3 → 4. Determine the total amount of work done by the gas and the heat supplied to the gas along each portion of the cycle.
Related questions
Question
100%
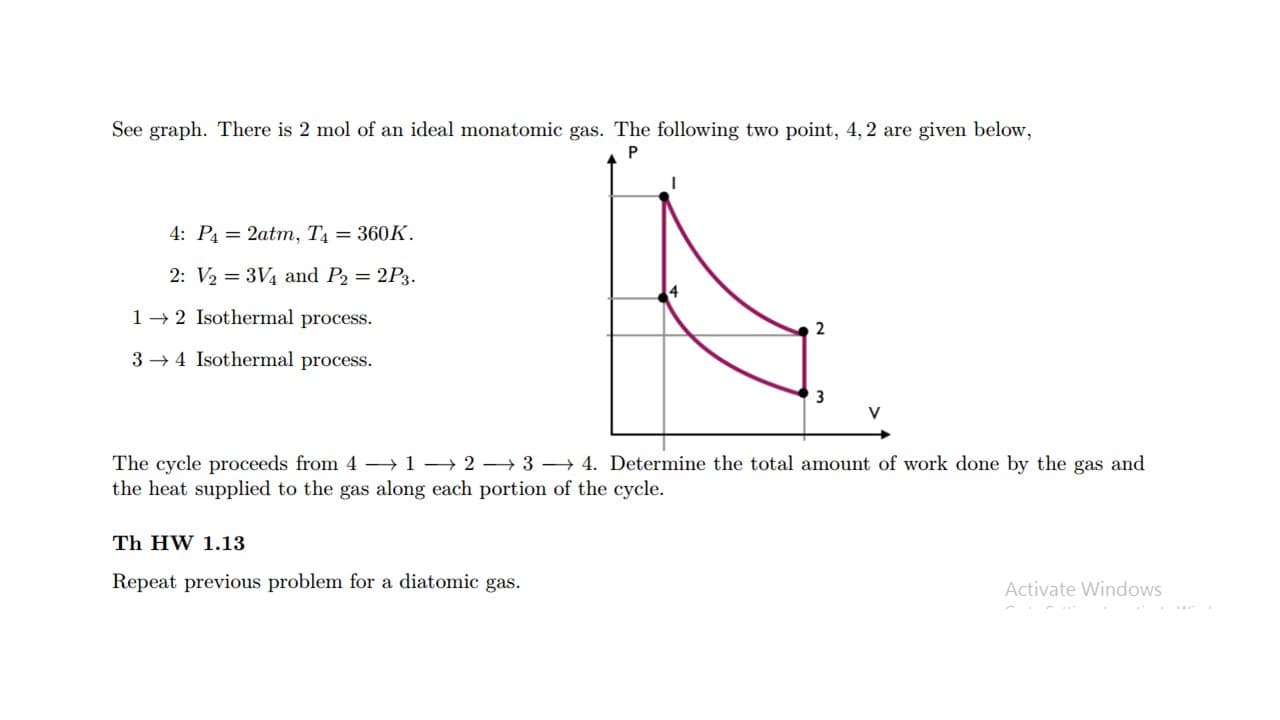
Transcribed Image Text:See graph. There is 2 mol of an ideal monatomic gas. The following two point, 4, 2 are given below,
4: P4 = 2atm, T4 = 360K.
2: V2 = 3V4 and P2 = 2P3.
1- 2 Isothermal process.
2
3 → 4 Isothermal process.
3
V
The cycle proceeds from 4 →1→ 2 – 3 → 4. Determine the total amount of work done by the gas and
the heat supplied to the gas along each portion of the cycle.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images
