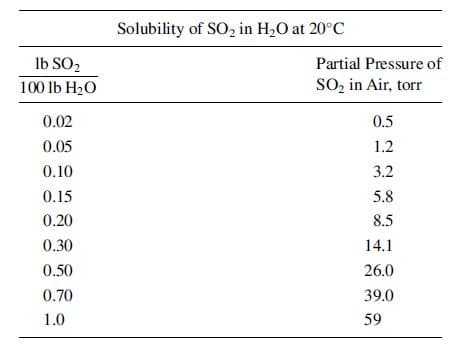
Solubility of SO, in H2O at 20°C Ib SO2 Partial Pressure of 100 lb H20 SO, in Air, torr 0.02 0.5 0.05 1.2 0.10 3.2 0.15 5.8 0.20 8.5 0.30 14.1 0.50 26.0 0.70 39.0 1.0 59
An SO2–air mixture is scrubbed with water in a packed tower at 20oC and 1 atm. Solute-free water enters the top at 1,000 lb/h and is well distributed over the packing. The liquor leaving contains 0.6 lb SO2/100 lb of solute-free water. The partial pressure of SO2 in the gas leaving is 23 torr. The mole ratio of water to air is 25. The necessary equilibrium data are given below. (a) What percent of the SO2 in the entering gases is absorbed in the tower? (b) In operation it was found that rate coefficients kp and kL remained substantially constant throughout the tower at:
kL = 1:3 ft/h
kp = 0:195 lbmol/h-ft2-atm
At a point in the tower where the liquid concentration is 0.001 lbmol SO2 per lbmol of water, what is the liquid concentration at the gas–liquid interface in lbmol/ft3? Solution density is 1 gm/cm3.
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 17 images









