Some N2 has is mixed with some O2 has and the sketch below shows a representative sample of the mixture. The total pressure of the mixture is measured and found to be 3.00 atm. Calculate the mole fraction and partial pressure of each gas in this mixture. Round your answer to 3 significant digits. You may assume each behave as an ideal gas
Some N2 has is mixed with some O2 has and the sketch below shows a representative sample of the mixture. The total pressure of the mixture is measured and found to be 3.00 atm. Calculate the mole fraction and partial pressure of each gas in this mixture. Round your answer to 3 significant digits. You may assume each behave as an ideal gas
Related questions
Question
Some N2 has is mixed with some O2 has and the sketch below shows a representative sample of the mixture. The total pressure of the mixture is measured and found to be 3.00 atm.
Calculate the mole fraction and partial pressure of each gas in this mixture. Round your answer to 3 significant digits. You may assume each behave as an ideal gas
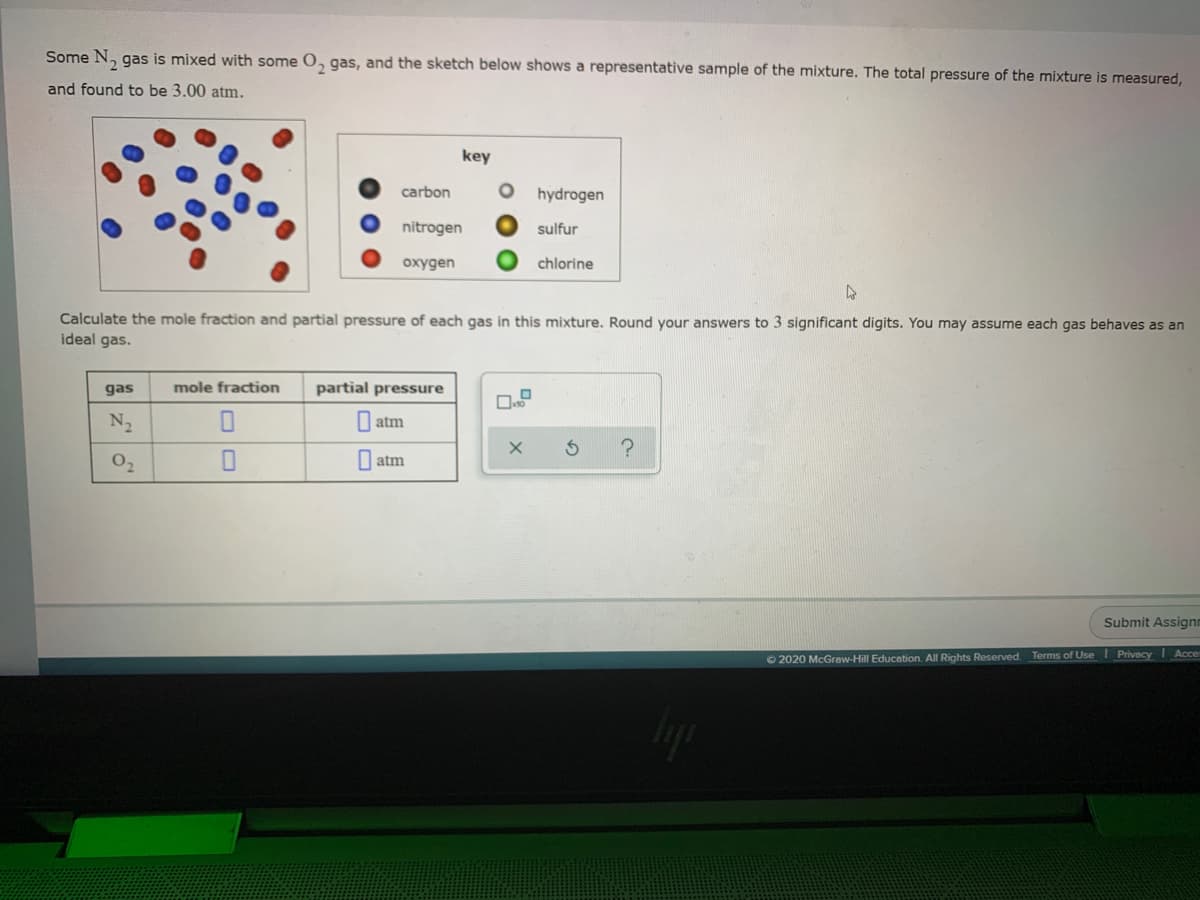
Transcribed Image Text:Some N, gas is mixed with some O, qgas, and the sketch below shows a representative sample of the mixture. The total pressure of the mixture is measured,
and found to be 3.00 atm.
key
carbon
hydrogen
nitrogen
sulfur
oxygen
chlorine
Calculate the mole fraction and partial pressure of each gas in this mixture. Round your answers to 3 significant digits. You may assume each gas behaves as an
ideal gas.
gas
mole fraction
partial pressure
N2
| atm
02
I atm
Submit Assignm
© 2020 McGraw-Hill Education. All Rights Reserved.
Terms of Use I Privecy I Acce
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 6 images
