Sort the following atoms in order of their first ionization potential from lowest to highest, that is from the easiest to hardest to remove one electron from the neutral atom. Dragged and dropped options will be automatically saved. For keyboard navigation... SHOW MORE ✓ E III E ||| ||| Question 10 E He (Z=2) Na (Z=11) O (Z=8) Ar (Z=18) Saved
Sort the following atoms in order of their first ionization potential from lowest to highest, that is from the easiest to hardest to remove one electron from the neutral atom. Dragged and dropped options will be automatically saved. For keyboard navigation... SHOW MORE ✓ E III E ||| ||| Question 10 E He (Z=2) Na (Z=11) O (Z=8) Ar (Z=18) Saved
Related questions
Question
Transcribed Image Text:(
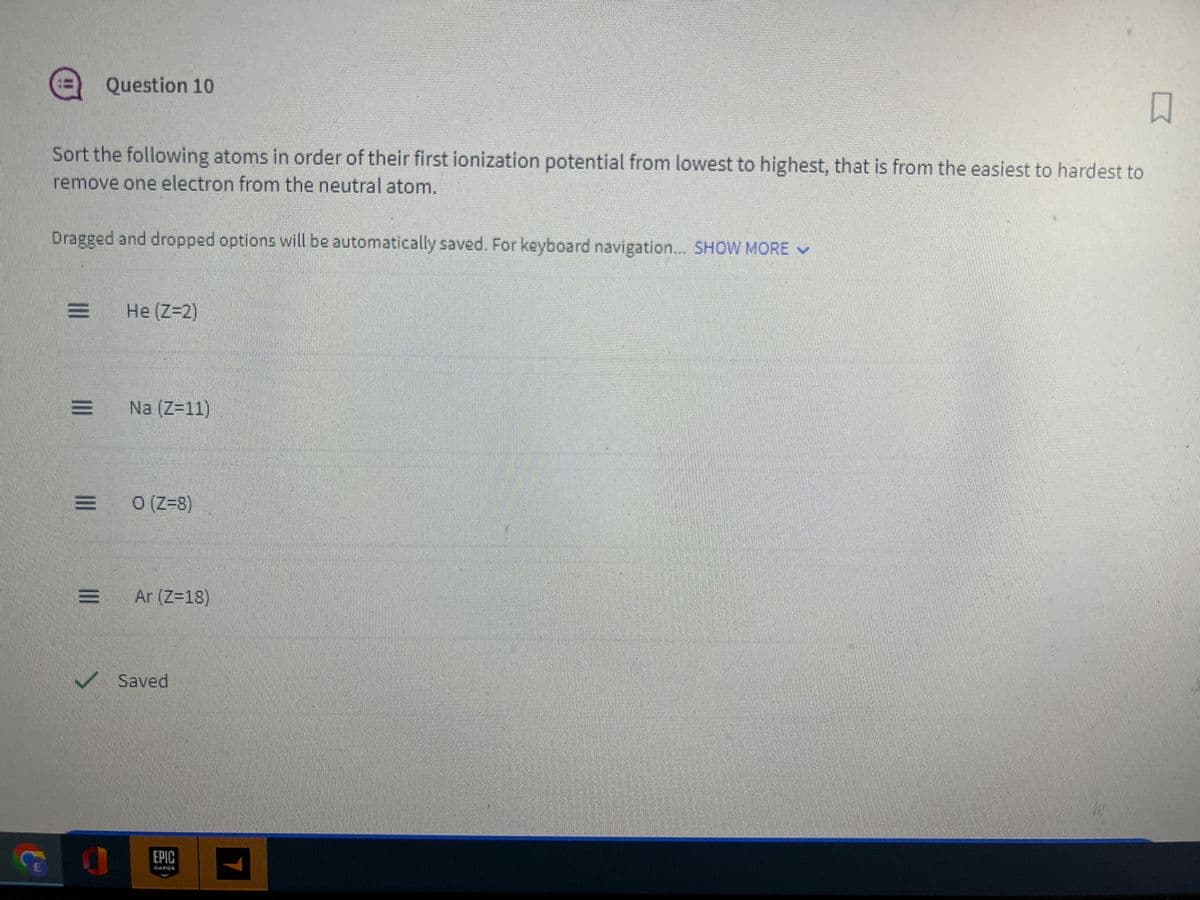
Sort the following atoms in order of their first ionization potential from lowest to highest, that is from the easiest to hardest to
remove one electron from the neutral atom.
Dragged and dropped options will be automatically saved. For keyboard navigation... SHOW MORE
IM
Question 10
III
III
He (Z-2)
Na (Z=11)
E 0 (Z=8)
= Ar (Z=18)
Saved
EPIC
L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps
