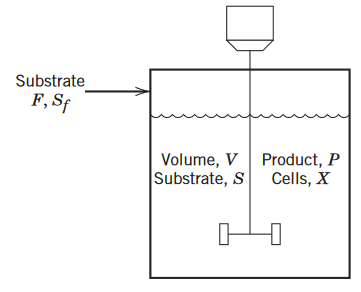
Substrate F, Sf Volume, V Product, P Substrate, S Cells, X
Suppose that the fed-batch bioreactor in Fig is con-verted to a continuous, stirred-tank bioreactor (also called achemostat) by adding an exit stream. Assume that the in let and exit streams have the same mass flow rate F thus the vol-ume of liquid V in the chemostat is constant.(a)Derive a dynamic model for this chemostat by modifying the fed-batch reactor model .(b)Derive the steady-state relationship between growth rate μ and dilution rate D where by definition,D=F/V.Suggest a simple control strategy for controlling the growth rate based on this result.(c)An undesirable situation called washout occurs when all of the cells are washed out of the bioreactor and thus cellmass X becomes zero. Determine the values of D that result in washout. (d)For the numerical values given below, plot the steady-statecell production ratevvDXbas a function of dilution ratebD. Dis-cuss the relationship between the values of D that result in washout and the value that provides the maximum produc-tion rate. The parameter values are:μm=0.20 h−1; KS=1.0 g/l,and YX/S=0.5 g/g. The steady-state condition is D=0.1h−1,X=2.25 g∕L,S=1.0g∕L, and Sf=10 g∕L.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images









