Table 2.1 Elements in the Human Body Percentage of Body Mass (including water) Element Symbol Oxygen 65.0% Carbon 18.5% 96.3% Hydrogen H 9.5% Nitrogen N 3.3% Calcium Ca 1.5% Phosphorus 1.0% Potassium K 0.4% Sulfur 0.3% 3.7% Sodium Na 0.2% Chlorine CI 0.2% Magnesium Mg 0.1% Trace elements (less than 0.01% of mass): Boron (B), chromium (Cr), cobalt (Co), copper (Cu), fluorine (F), iodine (0, iron (Fe), manganese (Mn), molybdenum (Mo). selenium (Se), silicon (S), tin (Sn), vanadium (M, zinc (Zn) INTERPRET THE DATA > Given the makeup of the human body. what compound do you think accounts for the high percentage of oxygen?
Table 2.1 Elements in the Human Body Percentage of Body Mass (including water) Element Symbol Oxygen 65.0% Carbon 18.5% 96.3% Hydrogen H 9.5% Nitrogen N 3.3% Calcium Ca 1.5% Phosphorus 1.0% Potassium K 0.4% Sulfur 0.3% 3.7% Sodium Na 0.2% Chlorine CI 0.2% Magnesium Mg 0.1% Trace elements (less than 0.01% of mass): Boron (B), chromium (Cr), cobalt (Co), copper (Cu), fluorine (F), iodine (0, iron (Fe), manganese (Mn), molybdenum (Mo). selenium (Se), silicon (S), tin (Sn), vanadium (M, zinc (Zn) INTERPRET THE DATA > Given the makeup of the human body. what compound do you think accounts for the high percentage of oxygen?
Concepts of Biology
1st Edition
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:Samantha Fowler, Rebecca Roush, James Wise
Chapter2: Chemistry Of Life
Section: Chapter Questions
Problem 7RQ: The pH of lemon juice is about 2.0, whereas tomato juice’s pH is about 4.0. Approximately how much...
Related questions
Concept explainers
Question
The percentages of naturally occurring elements making up the human body (see Table 2.1) are similar to the percentages of these elements found in other organisms. How could you account for this similarity among organisms?
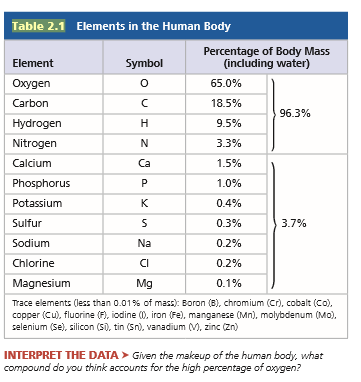
Transcribed Image Text:Table 2.1 Elements in the Human Body
Percentage of Body Mass
(including water)
Element
Symbol
Oxygen
65.0%
Carbon
18.5%
96.3%
Hydrogen
H
9.5%
Nitrogen
N
3.3%
Calcium
Ca
1.5%
Phosphorus
1.0%
Potassium
K
0.4%
Sulfur
0.3%
3.7%
Sodium
Na
0.2%
Chlorine
CI
0.2%
Magnesium
Mg
0.1%
Trace elements (less than 0.01% of mass): Boron (B), chromium (Cr), cobalt (Co),
copper (Cu), fluorine (F), iodine (0, iron (Fe), manganese (Mn), molybdenum (Mo).
selenium (Se), silicon (S), tin (Sn), vanadium (M, zinc (Zn)
INTERPRET THE DATA > Given the makeup of the human body. what
compound do you think accounts for the high percentage of oxygen?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Concepts of Biology
Biology
ISBN:
9781938168116
Author:
Samantha Fowler, Rebecca Roush, James Wise
Publisher:
OpenStax College

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781305073951
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology Today and Tomorrow without Physiology (Mi…
Biology
ISBN:
9781305117396
Author:
Cecie Starr, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Concepts of Biology
Biology
ISBN:
9781938168116
Author:
Samantha Fowler, Rebecca Roush, James Wise
Publisher:
OpenStax College

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781305073951
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology Today and Tomorrow without Physiology (Mi…
Biology
ISBN:
9781305117396
Author:
Cecie Starr, Christine Evers, Lisa Starr
Publisher:
Cengage Learning


Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781337408332
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning