Temp Vapor (°C) | Pressure vf Specific Volume |(m³/kg) Enthalpy (kJ/kg) hf Entropy (kJ/kg-K) sf y (kPa) V hv h % % Vv SV 82 80 52.5 67 2200 2500 14 4 180 145 33 6 2320 6.72 7 123 15 8 130 5.75 9 700.5 2500 10 115 1.005
Temp Vapor (°C) | Pressure vf Specific Volume |(m³/kg) Enthalpy (kJ/kg) hf Entropy (kJ/kg-K) sf y (kPa) V hv h % % Vv SV 82 80 52.5 67 2200 2500 14 4 180 145 33 6 2320 6.72 7 123 15 8 130 5.75 9 700.5 2500 10 115 1.005
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
Can you please help me, Can u please answer from 1-10 or just the hard one to solve from 1-10
here is the sample solution for item no.1
Please used this STEAM TABLE: https://drive.google.com/file/d/1hU6YI7iEsHYQqrnIHy8lLsEgqkpIRjqZ/view?usp=sharing
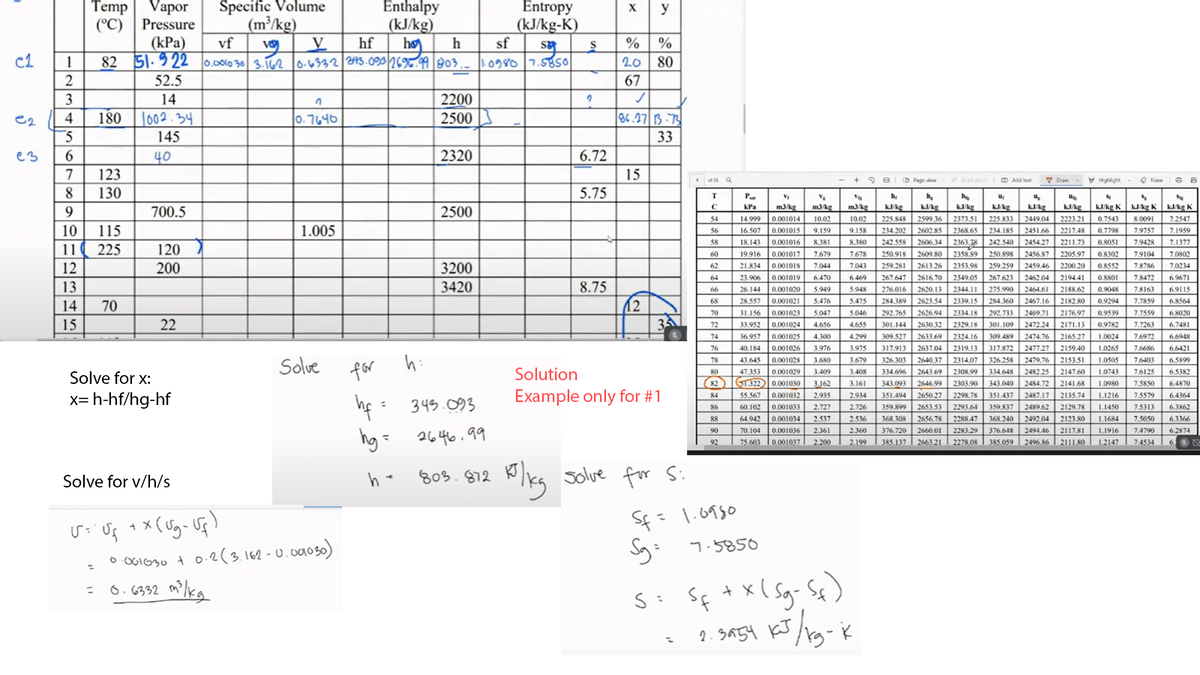
Transcribed Image Text:Specific Volume
(m³/kg)
vf
Temp| Vapor
Entropy
2123 s0
11684
Enthalpy
(kJ/kg)
h
X
y
(kJ/kg-K)
sf
(°C)
Pressure
% %
20 80
(kPa)
hf
hø
82 51.9 22 0.0010 36 3.162 0-4332 43.090269. 03.
c1
1
1og80 7.585o
2
52.5
67
3
14
2200
1602.34
145
e2
4
|0.7646
2500
86.27 B.73
33
40
2320
6.72
7
123
15
5.75
of 26 Q
2 BI D
D Page view
A ad
D Addtet
V Daw
Q Erase
8
130
P
kPa
m3/kg
m3/kg
m3/kg
kJkg
Kkg
kJ/kg
kJ/kg K kJ/kg K KJkg K
9
700.5
2500
kJ/kg
2223.21
54
14.999
0.001014
10.02
10.02
225.848
2599.36
2373.51
225.833
2449.04
0.7543
8.0091
7.2547
7.1959
10
115
1.005
56
16507
0.001015
9.159
9.158
234.202
2602.85
2368.65 234.185
2451.66 2217.48
0.7798
7.9757
58
18.143
0.001016
8.381
8.380
242.558
2606.34 2363. 242.540
2454.27
2211.73
0.8051
7.9428
7.1377
11
225
120
60
19.916
0.001017
7.679
7.678
250.918
2609.80
2358.89
250.898
2456 87
2205.97
0.8302
7.9104
7.0802
12
200
3200
62
21.834
0.001018
7.044
7.043
259.281
2613.26
2353.98
259.259
2459.46
2200.20
0.8552
7.8786
7.0234
23.906
26.144
6.470
267.647
2616.70
2349.05
2462.04
0.8801
7.8472
6.9671
6.9115
64
0.001019
6.469
267.623
2194.41
13
3420
8.75
0.001020
5.949
5.948
276.016
2620.13
275.990
2464.61
2188.62
0.9048
66
2344.11
7.8163
12
3
68
28.557
0.001021
5.475
284.389
2623.54
2339.15
284.360
2467.16
2182.80
0.9294
7.7859
6.8564
3.476
14
70
1.1859
70
31.156
0.001023
0.001024
5.047
5.046
292.765
2626.94
2334.18
292.733
2176.97
0.9539
7.7559
6.8020
S.046
4.655
5,047
292.135
2469.71
15
22
72
33.952
4.656
4.050
301.144
2630.32
2329.18
301.109
2472.24
2171.13
0.9782
7.7263
6.7481
2165.27
2159.40
74
36.957
0.001025
4.300
4.299
309.527
2633.69
2324.16
309.489
2474.76
2477.27
4.500
309 489
1.0024
1.0024
76972
7.6972
6.048
6.6948
76
40.184
0.001026
3.976
3.975
317.913
2637.04
2319.13 317.872
1.0265
7.6686
6.6421
h:
326.303
LOSOS
7.6403
Solue
78
43.645
0.001028
3.680
3.679
2640.37
2314.07
326.258
2479.76
2153.51
1.0505
65899
for
Solution
47.353
2308.99
334.648
2482.25 2147.60
2484.72
80
0.001029
3.409
3.408
334.696
2643.69
1.0743
7.6125
6.5382
Solve for x:
(82
S1322 0.001030
3.162
3.161
343.093
2303.90
7.5850
2646.99
2650.27
343.040
2141.68
1.0980
6.4870
x= h-hf/hg-hf
Example only for #1
84
55.567
0.001032
2.935
2.934
351.494
2298.78
351.437
2487.17
2135.74
L1216
7.5579
64364
hf :
343.093
86
60.102
0.001033
2.727
2.726
359.899
2653.53
2293.64
359.837
2489.62
2129.78
1.1450
7.5313
6.3862
64.942
0.001034
2.537
2.536
368.308
2656.78
228847
368.240
2492.04
2123.80
7.5050
63366
88
1.1684
2.361
70.104
75.603
2.360
2.199
2494.46
2496.86
376.720
2660.01
2663.21
2283.29
376.648
385.059
2117.81
L.1916
6.2874
7.4790
6
7.4534
90
0.001036
hg: 2646.99
92
0.001037
2.200
385.137
2278.08
2111.80
12147
803.872
Plkg solve for S:
Solve for v/h/s
Sf= 1.0930
7.5850
o O61030 t 0.2(3.162-0.0030)
= 6.6332
2. SA54 KJ
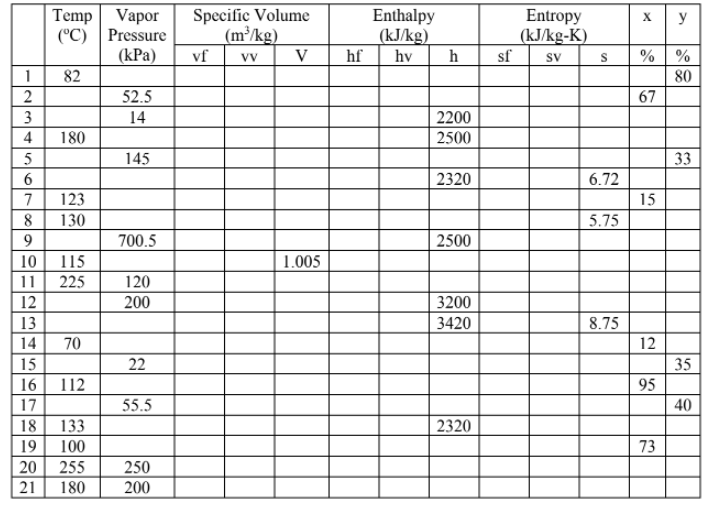
Transcribed Image Text:Specific Volume
(m³/kg)
V
Temp
Vapor
(°C) Pressure
vf
Enthalpy
(kJ/kg)
Entropy
(kJ/kg-K)
sf
y
(КРa)
hf
hv
h
% %
SV
82
80
52.5
67
3
14
2200
2500
4
180
5
145
33
6.
2320
6.72
7
123
15
8
130
5.75
9
700.5
2500
10
115
1.005
11
225
120
200
3200
3420
8.75
14
70
12
15
22
35
16
112
95
17
55.5
40
18
133
100
2320
19
73
20
255
250
21
180
200
2345 Or0 9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 8 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The