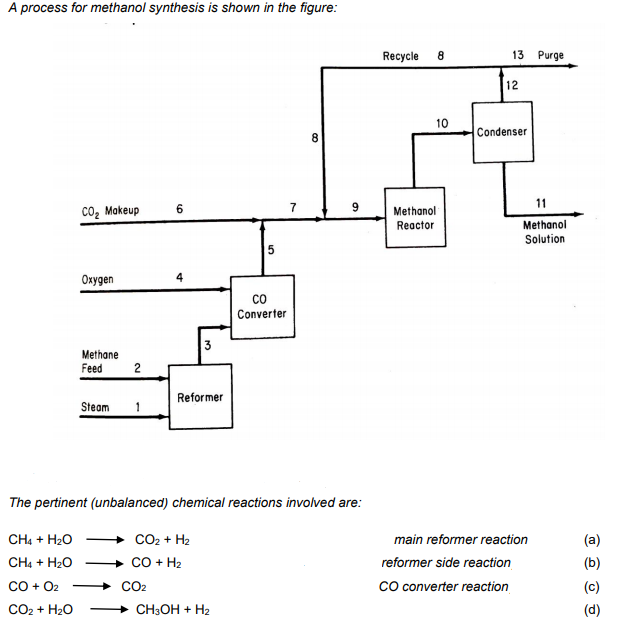
Ten percent excess steam, based on reaction (a), is fed to the reformer, and conversion of methane is 100%, with a 90% yield of CO2. Conversion in the methanol reactor is 55% on one pass through the reactor. A stoichiometric quantity of oxygen is fed to the CO converter, and the CO is completely converted to CO2. Additional makeup CO2 is then introduced to establish a 3-to-1 ratio of H2 to CO2 in the feed stream to the methanol reactor. The methanol reactor effluent is cooled to condense all the methanol and water, with the non-condensible gases recycled to the methanol reactor feed. The H2/CO2 ratio in the recycle stream is also 3-to-1. Because the methane feed contains 1% nitrogen as an impurity, a portion of the recycle stream must be purged, to prevent the accumulation of nitrogen in the system. The purge stream analyses 5% nitrogen. On the basis of 100 mol of methane feed (including the N2), calculate: (a) How many moles of makeup CO2 are required. (b) How many moles of H2 are lost in the purge. (c) How much methanol solution (in kg) of what strength (weight percent) is produced.
Ten percent excess steam, based on reaction (a), is fed to the reformer, and conversion of methane is 100%,
with a 90% yield of CO2. Conversion in the methanol reactor is 55% on one pass through the reactor.
A stoichiometric quantity of oxygen is fed to the CO converter, and the CO is completely converted to CO2.
Additional makeup CO2 is then introduced to establish a 3-to-1 ratio of H2 to CO2 in the feed stream to the
methanol reactor.
The methanol reactor effluent is cooled to condense all the methanol and water, with the non-condensible
gases recycled to the methanol reactor feed. The H2/CO2 ratio in the recycle stream is also 3-to-1.
Because the methane feed contains 1% nitrogen as an impurity, a portion of the recycle stream must be
purged, to prevent the accumulation of nitrogen in the system. The purge stream analyses 5% nitrogen.
On the basis of 100 mol of methane feed (including the N2), calculate:
(a) How many moles of makeup CO2 are required.
(b) How many moles of H2 are lost in the purge.
(c) How much methanol solution (in kg) of what strength (weight percent) is produced.
(d) The recycle to purge ratio in mol/mol.
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images









