The 2-carbon alcohol called ethanol is most common because it's made from something else very common: sugar! (this is also why ethanol is the one alcohol that's not toxic, our bodies can metabolize it). Ethanol is mass-produced in factories on an industrial scale, and is commonly used as a fuel (or mixed as an additive into other fuels). The combustion reaction below shows that 6 molecules of oxygen is required to completely burn two molecules of ethanol. How many molecules of carbon dioxide are produced after burning ethanol?
The 2-carbon alcohol called ethanol is most common because it's made from something else very common: sugar! (this is also why ethanol is the one alcohol that's not toxic, our bodies can metabolize it). Ethanol is mass-produced in factories on an industrial scale, and is commonly used as a fuel (or mixed as an additive into other fuels). The combustion reaction below shows that 6 molecules of oxygen is required to completely burn two molecules of ethanol. How many molecules of carbon dioxide are produced after burning ethanol?
Biology Today and Tomorrow without Physiology (MindTap Course List)
5th Edition
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cecie Starr, Christine Evers, Lisa Starr
Chapter13: Early Life Forms And The Viruses
Section: Chapter Questions
Problem 3SQ
Related questions
Question
100%
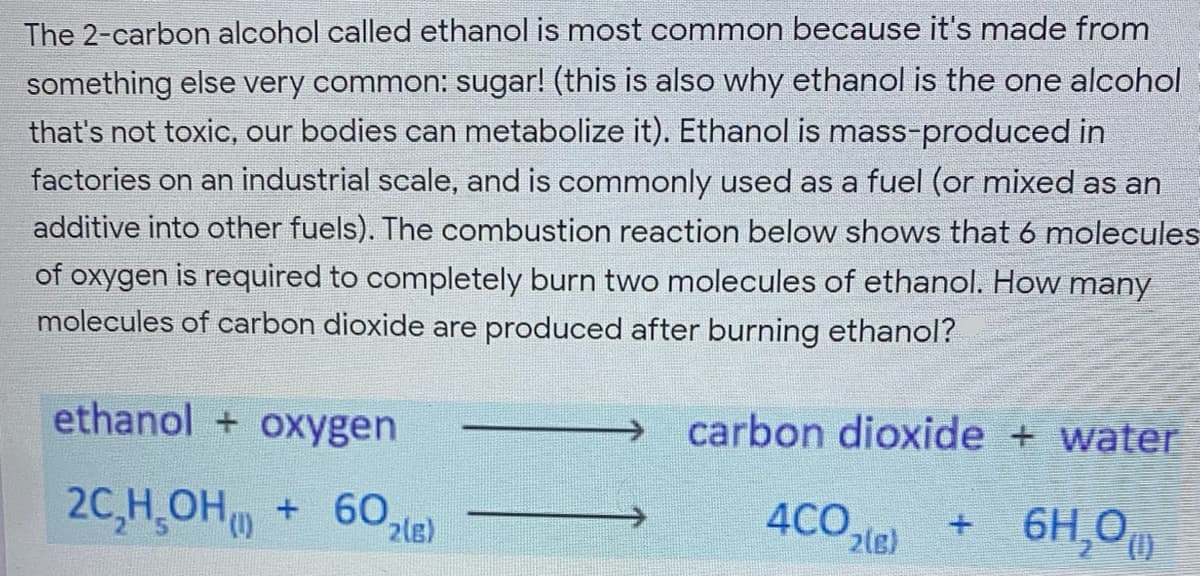
Transcribed Image Text:The 2-carbon alcohol called ethanol is most common because it's made from
something else very common: sugar! (this is also why ethanol is the one alcohol
that's not toxic, our bodies can metabolize it). Ethanol is mass-produced in
factories on an industrial scale, and is commonly used as a fuel (or mixed as an
additive into other fuels). The combustion reaction below shows that 6 molecules
of oxygen is required to completely burn two molecules of ethanol. How many
molecules of carbon dioxide are produced after burning ethanol?
ethanol + oxygen
carbon dioxide + water
20,H OH + 60,
+ 6H,0
4CO.
2(e)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biology Today and Tomorrow without Physiology (Mi…
Biology
ISBN:
9781305117396
Author:
Cecie Starr, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology Today and Tomorrow without Physiology (Mi…
Biology
ISBN:
9781305117396
Author:
Cecie Starr, Christine Evers, Lisa Starr
Publisher:
Cengage Learning