The addition of 5 grams of a compound to 750 g of CCl4 lowered the freezing point of the solvent by 10.5K. Calculate the molar mass of the compound.
The addition of 5 grams of a compound to 750 g of CCl4 lowered the freezing point of the solvent by 10.5K. Calculate the molar mass of the compound.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
The addition of 5 grams of a compound to 750 g of CCl4 lowered the freezing point of the solvent by 10.5K. Calculate the molar mass of the compound.
Use table for reference. thanks.
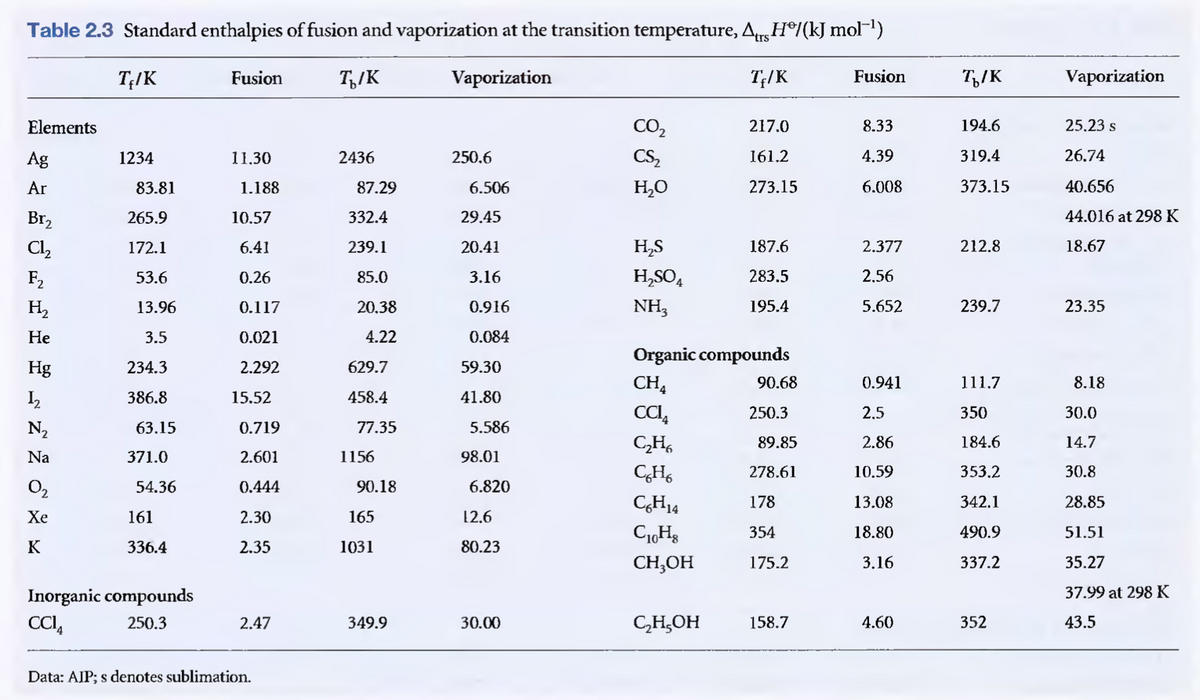
Transcribed Image Text:Table 2.3 Standard enthalpies of fusion and vaporization at the transition temperature, Atrg H®/(kJ mol")
T;IK
Fusion
T/K
Vaporization
T;/K
Fusion
T,/K
Vaporization
Elements
CO,
217.0
8.33
194.6
25.23 s
Ag
1234
11.30
2436
250.6
CS,
161.2
4.39
319.4
26.74
Ar
83.81
1.188
87.29
6.506
H,O
273.15
6.008
373.15
40.656
Br2
265.9
10.57
332.4
29.45
44.016 at 298K
Cl2
H,S
172.1
6.41
239.1
20.41
187.6
2.377
212.8
18.67
F,
53.6
0.26
85.0
3.16
H,SO,
283.5
2.56
H,
13.96
0.117
20.38
0.916
NH3
195.4
5.652
239.7
23.35
Не
3.5
0.021
4.22
0.084
Organic compounds
Hg
234.3
2.292
629.7
59.30
CH,
90.68
0.941
111.7
8.18
386.8
15.52
458.4
41.80
250.3
2.5
350
30.0
N2
63.15
0.719
77.35
5.586
CH,
89.85
2.86
184.6
14.7
Na
371.0
2.601
1156
98.01
CH6
278.61
10.59
353.2
30.8
O2
6.820
54.36
0.444
90.18
CH14
178
13.08
342.1
28.85
Хе
161
2.30
165
12.6
354
18.80
490.9
51.51
K
336.4
2.35
1031
80.23
CH,OH
175.2
3.16
337.2
35.27
Inorganic compounds
37.99 at 298 K
CCI,
250.3
2.47
30.00
CH,OH
158.7
4.60
352
43.5
349.9
Data: AIP; s denotes sublimation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The