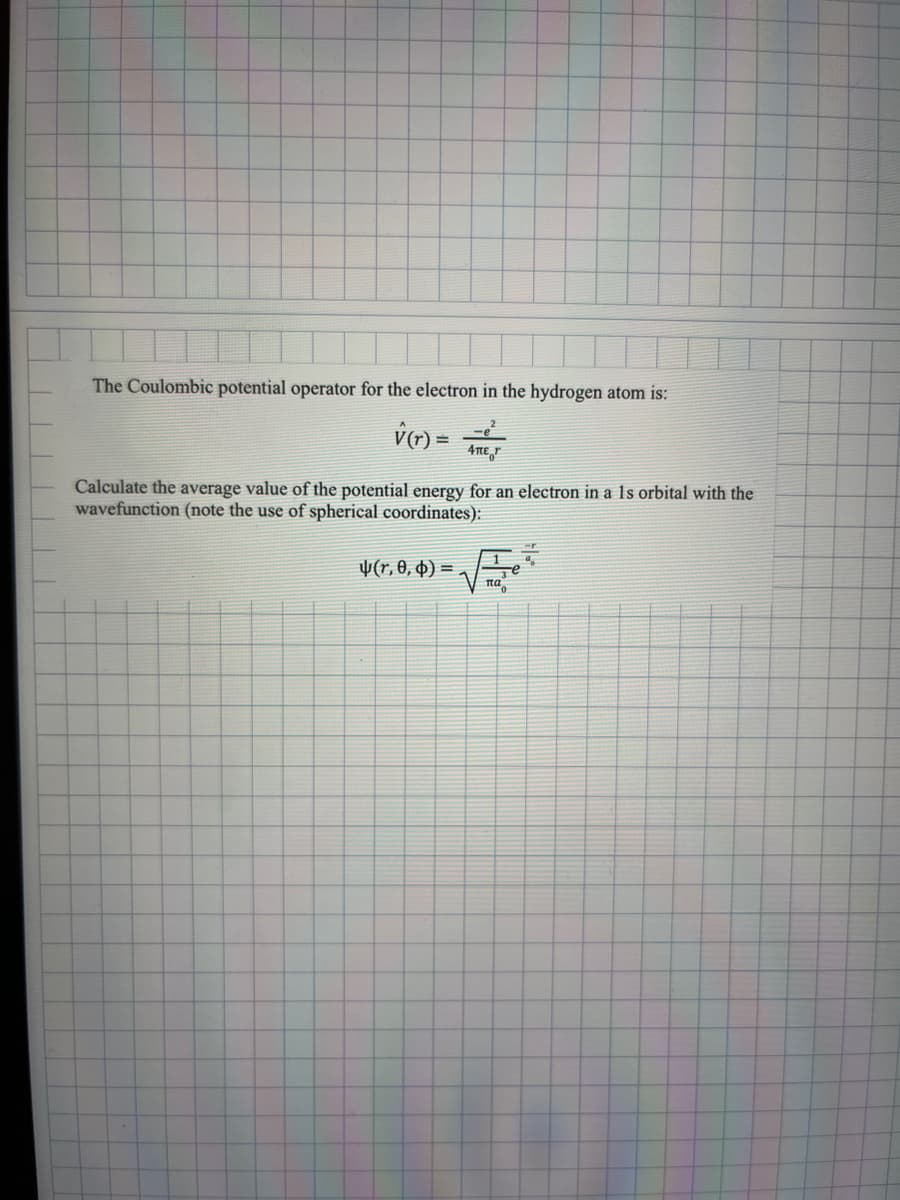
The Coulombic potential operator for the electron in the hydrogen atom is: V(r) = 4πer Calculate the average value of the potential energy for an electron in a 1s orbital with the vavefunction (note the use of spherical coordinates): √ (r, 0, 0). = πα
Q: The coefficient of friction between the inner pulley and the belt is 1/π. Find the maximum value of…
A:
Q: During a steady right turn, a person exerts the forces shown on the steering wheel. Note that each…
A: Solution: The moment exerted about the steering at O is given by: τ=FtRhere, Ft-tangential…
Q: A cylinder is half-filled with sand, sealed, and then thrown in the ocean. At equilibrium, it floats…
A:
Q: During a steady right turn, a person exerts the forces shown on the steering wheel. Note that each…
A: Answer:
Q: Task 1: ne = ke €0 = 10-⁹C 1.6-10-19 C 8.99 10⁹. 1 4. Ke N.m² C2 =?electrons 1 4T-E0
A: No of the electrons in a charge q is ne=qe=q1.6×10-19
Q: مد 92 حش شند 93 9a ه تیک ده ملا 92 9, له 9, )
A: In given question, resistors are connected in series and parallel connection. So we have to use…
Q: Q4) Your calculator gives you the following results. How will you round them and clearly present…
A: A good way to write the numbers in their correct significant figures. So before adding the results…
Q: 12) Calculate the maximum reflected wavelength by a thin film of refractive index 1.4 and thickness…
A: In a thin film, light waves are reflected from the background and the film is capable of interfering…
Q: Let C = A - B. Calculate the dot product of C with itself and thus derive the law of cosine
A:
Q: Lecture 4 - Homework problems: Show all work 1. Simplify then draw the following Boolean functions…
A:
Q: The current supplied to an air conditioner unit is 4.20 amps. The air conditioner is wired using a…
A: .
Q: 2. An inverted differential manometer is connected to two pipes A and B which convey water. The…
A:
Q: 3. An object is submerged in oil tank and tethered by a string to the lower surface of the tank. If…
A:
Q: Exercise I For two masses 1kg and 2kg, connected by three springs with stiffness 80, 40, 60. 1.…
A:
Q: (c) Argue that R v sin(20) would be applicable to the protons in this situation. 9 vsin(20) (d) Use…
A: This is a system based on the projectile motion of the proton. The projectile motion of the proton…
Q: Find the electric potential at a distance h from the center of a solid sphere of radius R, with a…
A:
Q: Calculate the threshold energy for the nt Th232 → Th²³1 + 2n 231
A:
Q: Two wiyes of the same material have their length in the ratio 3:2, diameter in ratio 3: and…
A: Given: The ratio of length is 3:2. The ratio of diameter is 3:2. The ratio of…
Q: The figure shows a thin, straight rod of length L which carries a charge which has a uniform, linear…
A:
Q: Quick description In this lab you will study two-dimensional motion, specifically projectile motion.…
A: The question contains more than 3 sub-parts. As per the Bartleby policy, we will answer the first 3.…
Q: (a) A bar of old (Au) is in thermal contact with a bar of silver (Ag) of the same length and area…
A: Given that the area and the length of both rod is same. Hence LAu= LAg And AAu = AAg
Q: What is the maximum kinetic energy of photoelectrons ejected from sodium by the incident radiation…
A: Given that-The wavelength of incident radiation is 450 nmPhotoelectric equation, ⇒hf= ϕ+ K.Ewhere, ϕ…
Q: A ban magnet of moment 7.9 Am² suspended in a uniform magnetic field of induction 3.6x 105 wb/m²…
A: We need to compute-Frequency of oscilations of bar (n)=?Given that-Magnetic moment (M)=7.9…
Q: Evaluate the expression A Xn-1 Xn-2 x2 x₁ n!ĵdx-1 [ dxn-z √ dx.-3...] dx₂ ¸ dx, ĵª dx, 0 0 0 0 0 0
A:
Q: The electric field a distance from a positive point charge is A vector pointing toward the…
A: Electric field at a point can be defined as force acting on unit positive test charge kept at that…
Q: A ring of charge is centered at the origin in the vertical direction. The ring has a charge density…
A: Given the linear charge density on the ring is λ=5.46×10-6C/m And the radius of the ring is…
Q: Each of the following vectors is given in terms of its x- and y-components. Find the magnitude of…
A: The magnitude of a vector in terms of its x and y components is given as r=x2+y2 And the direction…
Q: Do it asap
A: Given data: Mass of board, m=20 kg Mass of child sitting at a distance, x1=2.5 m from the pivot…
Q: 3. What is the equivalent resistance of the combination of identical resistors between points a and…
A: Given, R1=R2=R3=R4=R5=R R2, R3 and R4 are in parallel. 1RT=1R2+1R3+1R41RT=1R+1R+1R1RT=3RRT=R3
Q: Show complete solution. The rectangular coordinates of a particle which moves with curvilinear…
A:
Q: 11. If f(x) = cosh-1(5x), then f'(x) = 5 1 B) 5 √25x²+1 √√25x²+1 D) √25x²-1 12. Let f(x) =…
A:
Q: (c) Now consider a K+ ion (modelled as a single particle of mass 6.5 x 10-26 kg and charge +e) and a…
A:
Q: The stone, which has a mass of 0.16 kg, slides across the horizontal frozen pond. When it has a…
A:
Q: Write integrals that describe the x and y-components of the electric field at point P in each…
A: Solution for one rod System
Q: 400 cm w Figure P16.17) (c16p17) The three charges in Figure P16.17 are at the vertices of an…
A:
Q: Given x = ab² C3 if the percentage errors in a, b and care ± 1%, ± 3% and ± 2% respectively, the…
A: Given data, Function is given as, x=ab2c3 Percentage errors in a b and c are ±1%,±2% and ±3%
Q: What's the difference between the Instrumental Theories and Classical Theory?
A: Difference between the Classical Theory and Instrumental Theory is given below
Q: In the figure positive charge q - 8.00 pC is spread uniformly along a thin nonconducting rod of…
A: Solution:-Given thatq=8 pC=8×10-12 CL=16 cm=0.16 mR=7 cm=0.07 m
Q: A projectile is launched from point A with the initial conditions shown in the figure. Determine the…
A:
Q: Explain in your own words why did the puffed wheat cereal, in the movie, fly off.
A: Puffed wheat cereal is an excellent illustration of electrostatic repulsion. This phenomenon is…
Q: Why does a spoon appear bent when it's in a glass of water?
A: The speed of light is the fastest in the universe. No objects with mass are capable of reaching the…
Q: 2. Determine the power of the current source (include whether it is supplying or absorbing power).…
A:
Q: An experimental device imparts a force of magnitude F = 41 lb to the front edge of the rim at A to…
A: Given data: Distance between point B and the edge of the net, b = 12 inch Distance between point B…
Q: Two rods and one cable are attached to the supports at O. If two of the forces are as shown,…
A:
Q: Q2) You time how long it takes for a toy car to roll down a ramp with your smartphone and find it…
A: Every instrument has error. The least count is the least error fond in any instrument. So every…
Q: Q6) Two quantities are measured with uncertainties, as follows: r = 5.6 ± 0.3 Calculate the values…
A: Since you have posted a question with multiple sub-parts, we will solve the first three sub-parts…
Q: The deceleration of the mass center G of a car during a crash test is measured by an accelerometer…
A:
Q: The Van der Waals interaction energy between two spherical nanoparticles of diameters D₁ and D2, a…
A:
Q: Assume that a GaAs semiconductor has a lattice constant a = 5 Å, and the atoms of Ga and As are hard…
A:
Q: Suppose you walk 18.0 m straight west and then 25.0 m straight north. How far are you from your…
A: given that first walking displacement in the west A = 18.0 m and the second displacement in the…
Step by step
Solved in 3 steps with 3 images
