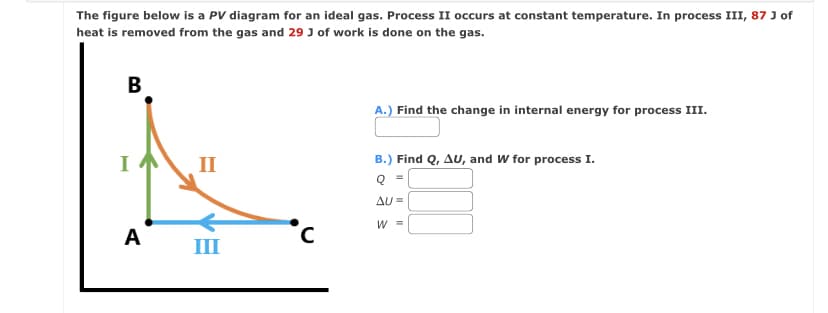
The figure below is a PV diagram for an ideal gas. Process II occurs at constant temperature. In process III, 87 J of heat is removed from the gas and 29 J of work is done on the gas. B = ^ A II III A.) Find the change in internal energy for process III. B.) Find Q, AU, and W for process I. = AU= W =
Q: 2. A satellite orbiting the Earth is powered by photovoltaic ("solar") panels that convert light…
A: Given that: Pout=3.60 kWPout=0.15 PinSolar power=Ps(Let)Let the temperature of the surface of the…
Q: Given the voltage sources e₁=20 V, e2=5 V, and the resistances R₁=1000, R₂-3000 and R3=5002, find…
A:
Q: A glass cylinder filled with n = 0.91 mol of air is capped with a moveable piston that can slide…
A: Given:number of moles of air, n = 0.91 constant force, F = 190 Ndisplacement of the piston, d = 14…
Q: The quarter ring shown has a mass 4kg and was cut from a thin, uniform plate. Knowing that r₁ = 80mm…
A:
Q: The temperature coefficient of resistivities of three different materials are listed. The said…
A: Given:length, area and resistivity of 3 materials is same at To = 20 oCtemperature is raised from…
Q: arged to 36
A: Given: Charge up to 36 % final charge
Q: A Nichrome heater dissipates 510 W when the applied potential difference is 110 V and the wire…
A:
Q: Each of two long straight parallel wires 8 cm apart carries a current of 125 A. The figure shows a…
A: We are given 2 long straight wires. We know that magnetic field due to straight wire at a point is…
Q: Consider the father pushing a playground merry-go-round in CNX_HSPhysics_06_03_MerryGo. He exerts a…
A: Given that:-→Force exerts by fatherF=250N→Radius of merry-go-roundr=1.50m→Torqueτ formula is given…
Q: Given two particles with Q = 2.30-µC charges as shown in the figure below and a particle with charge…
A:
Q: A proton is shot between 2 charged plates as seen below. Which direction would a magentic field…
A:
Q: A spherical conductor with a 0.493 m radius is initially uncharged. How many electrons should be…
A:
Q: A bicycle has a pedal sprocket with radius 6 inches, a tire sprocket with radius of 2 inches, and a…
A:
Q: You're considering purchasing a new sleeping bag whose manufacturer claims will keep you warm to…
A: Given that the bag's thickness is 4.0 cm, the rate at which the body generates heat is 100 W, and…
Q: person pu Eceleration
A: Given: Linear acceleration is 3 m/s2 Radius or width of door is 0.5 m
Q: The cylindrical coordinates of a point P are (10, 60°, 5) The rectangular coordinates are
A:
Q: 5. A 5kg mass is located on a wedge angled 30° above the horizontal, with a string connected to a…
A: Given data, block diagram is given as, Mass is given m=5 kg. Wedge angle θ=300. Force F = 30 N.
Q: 2. A1m length dye laser emitting around 500 nm emits 500 longitudinal modes. What is the bandwidth…
A:
Q: Kennedy is going to throw a ball from the top floor of the Stark building in San Diego. The function…
A: h(t) = -16t 2 + 16 t +320 When ball reached the ground floor h(t) will be zero Which will give us…
Q: A rectangular trough, 2.0 m long, 0.60 m wide, and 0.45 m deep, is completely full of water. One end…
A: Given a rectangular system with, Length of rectangle, l = 2 m Width of rectangle, w = 0.6 m Depth of…
Q: Ex. 63: A motor car is travelling at a speed of 80 km/hr along a curve of radius 40 m. If the road…
A: We have to check-Whether the speed of car is sufficient for safety of car, or not.The data given…
Q: 13. Like a Hurricane. Four charged particles of equal magnitude are placed equidistant from point P…
A: Disclaimer: “Since you have asked multiple question, we will solve the first question for you. If…
Q: Each of the plates of a parallel-plate capacitor has an area of 2.00 cm², and they are separated by…
A: Given,The area of a parallel plate,A = 2 cm2=2×10-4 m2The distance between the plates, d = 2.5…
Q: The amplitude of two beating waves is described by the equation ψ = Amod(t)cos (ωavt). What is the…
A:
Q: 3/2 v²e-Mv2/2R derive the expression for (a) vavg, (b) (v²) avg, (c) M Starting with (v) = 4T 2πRT…
A:
Q: You are creating a semiconduxtor (hetero structurre) with a depth of 0.29 eV. Suppose well width is…
A:
Q: it take t
A: Given: Distance is 6.2 nm New distance can be 3.57 nm
Q: Prove that the expectation value of the potential energy in the nth state of harmonic oscillator is:…
A: According to classical physics, a harmonic oscillator is a body exerted by a force proportional to…
Q: A proton moves perpendicular to a uniform magnetic field B at a speed of 1.20 x 107 m/s and…
A:
Q: a 45 m wire is coiled so that it makes a coil containing 100 circular loops, one on top of the…
A: Given: In this question, the given values are, The length value is, L=45 m. The given value of the…
Q: Eure (N/m¹?) g of gasoline b
A: Given: Width is 0.541 m Length is 0.834 m Mass of gasoline is 55.7 kg
Q: a) Why transformers cannot work with Direct Current? b) What are the major losses that should be…
A: A transformer is a device which works on electromagnetic induction. When an alternating current is…
Q: Consider the two Lorentz invariants EB and c²B2- E². (a) Suppose that in one inertial frame E B = 0…
A:
Q: Ordinary X-ray photographs cannot produce sharp images of internal organs whose densities are not…
A: When an ordinary X-ray is used for scanning internal organs we don't get a sharp image of the…
Q: 5. As a wire loop rolls along a lab table, it starts off in a region with no magnetic field, enters…
A: We are given a circular loop. This loop is rolling. It comes inside magnetic field which is uniform…
Q: v/s v/s
A: Given: Moment of inertia is 10 kgm2 Initial angular speed is 6 rev/s Final moment of inertia is 15…
Q: 1. A 5.0000 x 10-kg subway train is brought to a stop from a speed of 0.6000 m/s in 0.3000 m by a…
A: Given, A subway train of mass m=5.00×105kg moving with a speed vi=0.600 m/s is brought to rest in…
Q: An elevator cab and its load have a combined mass of 1600 kg. Find the tension in the supporting…
A:
Q: A spring with a 10-kg mass and a damping constant 18 can be held stretched 0.5 meters beyond its…
A:
Q: 6. Which one is the optical path length between points A and C? a) d₁.sec 01 + d₂.sec 02 b) d₁.cosec…
A: Given data, The path of light travel is,
Q: A transmission line strung 9 m above the ground carries a current of 400 A. What is the magnetic…
A: Answer)
Q: A negative point charge is moved along each of the paths shown. In which of the following paths is…
A:
Q: by a moving belt. Charge can be added until the electric field at the surface of the dome becomes…
A: Solution: a). The maximum potential can be written in terms of an electric field as given below,…
Q: Two taut ropes, each 1.2 m long, support standing waves. On the first rope, there is a second…
A: Given data, Length of rope l = 1.2 m. 1st harmonic frequency ν1=600 Hz. 3rd harmonic frequency…
Q: A parachutist is falling with speed of 55 m/s when his parachute opens. If the air resistance O a.…
A: We have to find the speed of man at t=10 s
Q: sed at t = C
A: Given: Potential difference is 75 V Potential difference across capacitor is 1.10 V Initial time is…
Q: You are working during the summer at a company that builds theme parks. The company is designing an…
A: It is given that, length of rod is, d=1.00 m, mass of rod is, m=0.60 kg, current in rod is, I=100 A,…
Q: • Using the notation 40 for angular displacement, 0 for angular position at time t, 0, for the…
A: Disclaimer: “Since you have asked posted a question with multiple sub-parts, we will solve the first…
Q: If you place 0°C ice into 0°C water in an insulated container, what will happen? Will some ice melt,…
A: Thermal conductors are those materials that will allow heat energy to pass through, and thermal…
Q: 1. A piece of dust finds itself on a CD. The spin rate of the CD is omega rpm, and the piece of dust…
A: We are given the the radius of dust. We are given the angular velocity. We are given the time till…
Step by step
Solved in 2 steps with 2 images
