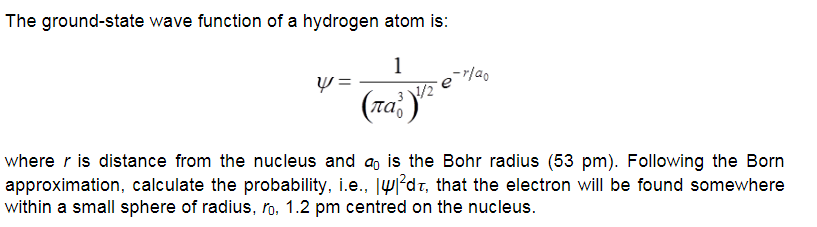
The ground-state wave function of a hydrogen atom is: 1 where r is distance from the nucleus and a, is the Bohr radius (53 pm). Following the Born approximation, calculate the probability, i.e., Jw°dz, that the electron will be found somewhere within a small sphere of radius, ro, 1.2 pm centred on the nucleus.
Q: 16. The spin, I of ground state for even-even nucleus is zero. But for odd nuclei, I 0. for example ...
A:
Q: Q3: Calculate the minimum energy required to remove an alpha particle from the 238 Uranium isotope U...
A: An isotope of Uranium decays to Thorium via the process of alpha decay. The reaction is as shown bel...
Q: Charge is distributed along the entire x axis with uniform density λx and along the entire y axis wi...
A: Given: The uniform charge distributed along the x-axis is λx The uniform charge distributed along t...
Q: Ball 1 is launched with an initial vertical velocity v = 142 ft/sec. Ball 2 is launched 2.3 seconds ...
A: Given, at time t = 0s, the initial velocity of ball 1 is, v1=142ft/s at time t = 3s, the initial vel...
Q: A solid sphere made of conductive material, radius a has a positive charge amount of 20. A spherical...
A:
Q: s are in tens
A: The given structure is equal to the below equation as, By =cy =2+2+4+42 =6kN
Q: 8. The trumpet player now pushes both the 1ª and 2nd valves, opening both bits of extra tubing. What...
A: Given information: The velocity of the sound (v) = 343 m/s The frequency of the 6th harmonic when no...
Q: A string of Christmas decoration lights consists of nine 24-W, 22-V bulbs connected in series. What ...
A:
Q: 4- The CO2 laser has a wavelength of 10.6 um and a spherical resonator length of 10 cm. The magnitud...
A: Given,wavelength λ=10.6μmResonator length L=10cm
Q: Typical backyard ants often create a network of chemical trails for guidance. Extending outward from...
A: Given Length (l) = 1.9 cm Angle θ = 30° Find the magnitude and angle points A & B, as following ...
Q: A particle of spin 1/2 and a particle of spin 1 are in configuration with total spin equal to 3/2 an...
A: Given, the spin of the two particles are 12 and 1. For the spin 1 particle, its z components are 1,0...
Q: ди = k ôt Solve the heat equation: 00, subject to boundary conditions: (0,t) = 0 and ôx (L,t) = 0 fo...
A: The heat equation is given as : ∂u∂t=k∂2u∂x2 Solving the heat equation : Let us consider :u(x,t)=F(x...
Q: If the light intensity is doubled by 210 after passing through a laser amplifier of length 14 cm, th...
A:
Q: A Co2 laser has a Wavelength of lenGum and a spherical re sona dor length of locm, So what is the ma...
A:
Q: A sphere made of an insulating material, and which has a radius a = 0.5 m, has a uniformly distribut...
A:
Q: The equations below describe the location of a package sliding down in a ramp, where t is in seconds...
A: The magnitude of the acceleration of the package along the x, y, and z-axis at t=2s is : x=0.25t3x'=...
Q: An object has a density of 5.6 x 105 kg/m³. What is the mass of the object if its volume is 4.5 x 10...
A:
Q: A proton, with a velocity of 3.4 x 10°m/s.enters a magnetic field perpendicular to the field lines. ...
A: Given: The velocity of proton enters into magnetic field is v = 3.4 x 106 m/s The magnetic field int...
Q: Resolution is defi
A: The smallest things can be observed largest by using devices,the process is called as resolution.
Q: shows electrons 1 and 2 on an x axis and charged ions 3 and 4 of identical charge −q and at identica...
A: Two charges placed close to each other will experience a force given by, F=kq1q2r2 Here q1, q2 are t...
Q: two speakers are separated along a straight line on the x axis. both speakers are fed by a single so...
A: Given: The frequency of the two speakers is f = 300 Hz The speed of sound in air is v = 343 m/s
Q: On a hot day in the desert (T = 46 °C), a sound of 496 Hz is emitted by a drone travelling at 139 km...
A:
Q: Detemine the force in element CE and state If the member is In tenslon or compression 10 m 5 m to to...
A: Draw the free body diagram as below. Calculate of reaction, ∑M=0VE×8 m=509.3 N×8+8mVE=1018.6 N
Q: A solid disk is initially at rest and then is spun for a time of s seconds with a torque of T, cover...
A: 3a) The torque acting on the solid disc can be formulated by the following equation, τ=Iατ=Torque a...
Q: e Earth as represente-
A: Given as, ωE =360 degday
Q: An insulating sphere with radius R = 10 cm has a charge uniformly distributed in its volume equal to...
A: Given: Radius, R=10 cm Charge, q=5 nC To find: Electrostatic field at distance 5 cm from the center ...
Q: In the figure particle 1 (of charge +9.37 mC), particle 2 (of charge +9.37 mC), and particle 3 (of c...
A: Given, Q1= 9.37 mCQ2= 9.37 mCQ3= Q
Q: e pilot of an airplane carrying a package of mail to a remote outpost wishes to release the package ...
A: Given: The velocity is 223 km/h. The height is 158 m. To determine: The angle, θ. The p...
Q: The electric potential created by a charge distribution is V = ax + By + yz where a, B and Y are con...
A: Given, V=αx+βy+γz
Q: The mass M and moment of inertia C of a thick shell of uniform density p, with internal radius r and...
A: Given, The radius and density of the different layers of the earth is Layer Radius Density kg/m3...
Q: For the circuit shown below, a) find the load resistance, RL, to obtain maximum power transfer in th...
A: (a) For the maximum power transfer, the load resistance should be equal to the Thevenin resistance. ...
Q: The equation below describes the position of a particle in a rectilinear path, where x is in seconds...
A: Here we have a easy question. First we have to find the velocity and we have to check the nature of ...
Q: 1. Consider a receiver with a 4-dB noise figure and a 2-MHz bandwidth. If a carrier-to-noise ratio o...
A:
Q: two speakers are separated along a straight line on the x axis . both speakers are fed by a single s...
A: Sound travels as waves. It will have a minimum and maximum amplitude is for a crest a minimum is for...
Q: The z-transform of x[n] = u[-n]
A:
Q: What is the energy required to remove the least tightly bound neutron from Ca-40? (Mca-40 = 39.96258...
A:
Q: Q2:- Calculate the initial velocity of proton under the head-on collision with a gold nucleus 19,Au ...
A: When a charged particle such as a proton is headed for a head on collision with some nucleus at rest...
Q: n observatory ne star? At th
A: We have the following relation : Declaration=elevation-(900-latitude) elevation=340Latitude=420 Dec...
Q: Prove that V. (pū) = pV · J+ ở · Vp Prove that V. (ũ + 0) = · ū+ ỹ · J.
A: To prove the identities: 1) ∇.(ρv→)=ρ∇.v→+v→∇ρ2)∇→.(u→+v→)=∇→.u→+∇→.v→
Q: V12 m2 V1 V2 Ptotal |-0.36kg F0.15 ms 3.96 kgm/s O7b mls 1.20 kg 1.20 kg +1.50 m/s -1.80 m/s 2.40 kg...
A: The problem is based upon the law of conservation of momentum. To solve we have to use the law of co...
Q: turns green. After B) 16 C) 8 D) 12 E) 6 2) A ball is dropped from a window 60m above the ground. Th...
A: (2) Given Data The displacement of the drawn ball is :s=60 m The acceleration due to gravity is:a=9...
Q: Determine the maximum speed for each car if the normal acceleration is limited to 0.84g. The roadway...
A:
Q: If o = (r x a) (r x b), show that Vọ = bx (r x a) + ax (r x b)
A: Given, ϕ=r×a·r×b Let vectors are r=xi^+yj^+zk^a=a1i^+a2j^+a3k^b=b1i^+b2j^+b3k^ Then r×a=xi^+yj^+zk^×...
Q: Point charges q, = -2.97 nC and q, = +6.29 nC are located on the x-axis and separated by a distance ...
A: Given: Charge 1,q1=-2.97nC=2.97×10-9CCharge 2,q2=6.29nC=6.29×10-9CTotal Distance,d=65cm=65×10-2mDist...
Q: An infinite plane, placed in a vacuum, has a surface charge density, uniform throughout the plane, e...
A: Given,Surface charge density =σ
Q: A 3,000-kg truck leaves a freeway on a circular exit of radius 50 m at a speed of 15 m/s. What minim...
A:
Q: 2)The eftect of 8timu emissionson the Change of Plane count and EE Can be expressed b3theeguatien )d...
A: Spontaneous emission is jump of electron from higher excited state to lower ground state by emitting...
Q: At what temperature will silver have a resistivity that is four times the resistivity of copper at r...
A: It is asked to find at what temperature silver have a resistivity that is four times the resistivity...
Q: Find the mechanical moment acting on an electrostatic dipole equal to P = p.(i+ 2j) immersed in a fi...
A: Vector is an abstract concept that is used to describe a quantity that has two attributes, ie magnit...
Q: Cite five examples on how relativity is applied and what is its use. Example: GPS (Global Positioni...
A: The real-life examples which are based on relativity are : Electromagnets - When a current is passe...
Trending now
This is a popular solution!
Step by step
Solved in 3 steps
