The internal energy U of a system is given by U (S,V)= AV2/s², where a is a constant of appropriate dimensions; V and S denote the volume and entropy, respectively. Which one of the following gives the correct equation of state of the system? PV/3 (a) T? PV = constant = constant (b) PV 2/3 (c) = constant (d) T T/3 = constant
The internal energy U of a system is given by U (S,V)= AV2/s², where a is a constant of appropriate dimensions; V and S denote the volume and entropy, respectively. Which one of the following gives the correct equation of state of the system? PV/3 (a) T? PV = constant = constant (b) PV 2/3 (c) = constant (d) T T/3 = constant
Related questions
Question
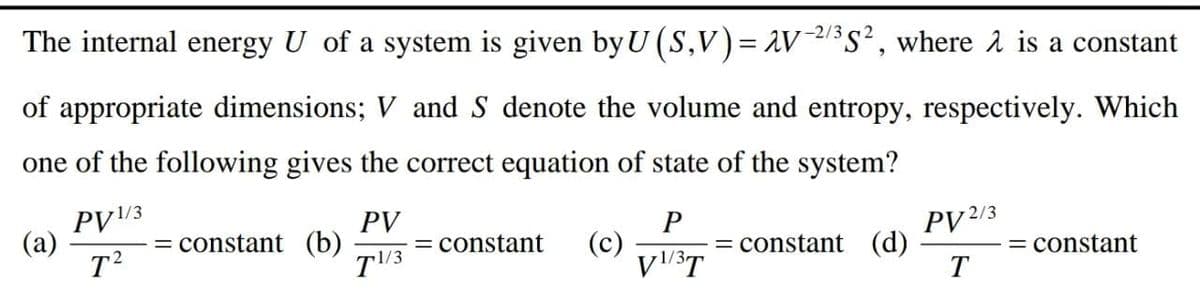
Transcribed Image Text:-2/3
The internal energy U of a system is given by U (S,V)= AV/s², where å is a constant
of appropriate dimensions; V and S denote the volume and entropy, respectively. Which
one of the following gives the correct equation of state of the system?
PV/3
(а)
T2
PV
PV2/3
= constant (b)
= constant
T3
(c)
= constant (d)
T
= constant
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images
