The lime dose necessary (mg/L as CaO) to soften this water if the lime is 90% pure is most nearly? You may assume excess lime needed is 20 mg/L as CaCO3 Concentration (mg/L as СаСОз) Compound CO2 5 Ca2+ Mg2+ HCO3 300 30 250 SO42- 171 mg/L 80 159 mg/L 142 mg/L 283 mg/L
The lime dose necessary (mg/L as CaO) to soften this water if the lime is 90% pure is most nearly? You may assume excess lime needed is 20 mg/L as CaCO3 Concentration (mg/L as СаСОз) Compound CO2 5 Ca2+ Mg2+ HCO3 300 30 250 SO42- 171 mg/L 80 159 mg/L 142 mg/L 283 mg/L
Traffic and Highway Engineering
5th Edition
ISBN:9781305156241
Author:Garber, Nicholas J.
Publisher:Garber, Nicholas J.
Chapter18: Bituminous Materials
Section: Chapter Questions
Problem 4P
Related questions
Question
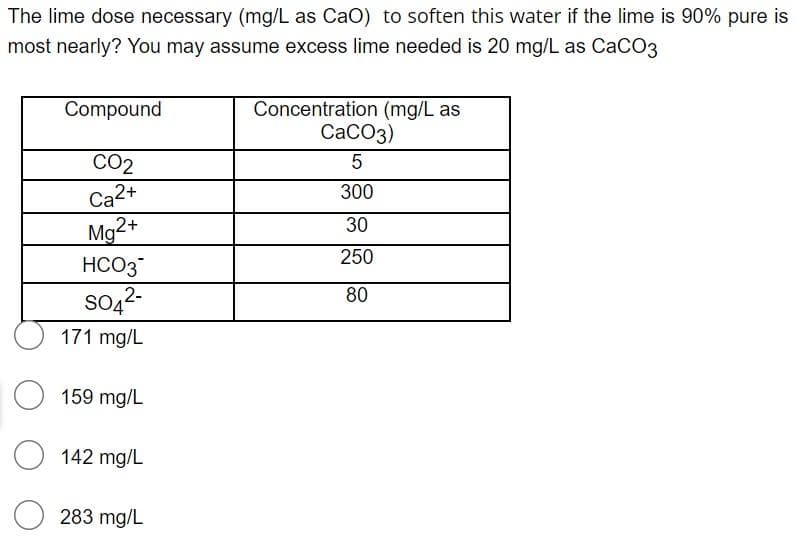
Transcribed Image Text:The lime dose necessary (mg/L as CaO) to soften this water if the lime is 90% pure is
most nearly? You may assume excess lime needed is 20 mg/L as CaCO3
Concentration (mg/L as
СаСОз)
Compound
CO2
5
Ca2+
Mg2+
HCO3
300
30
250
SO42-
171 mg/L
80
159 mg/L
142 mg/L
283 mg/L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Traffic and Highway Engineering
Civil Engineering
ISBN:
9781305156241
Author:
Garber, Nicholas J.
Publisher:
Cengage Learning

Solid Waste Engineering
Civil Engineering
ISBN:
9781305635203
Author:
Worrell, William A.
Publisher:
Cengage Learning,

Traffic and Highway Engineering
Civil Engineering
ISBN:
9781305156241
Author:
Garber, Nicholas J.
Publisher:
Cengage Learning

Solid Waste Engineering
Civil Engineering
ISBN:
9781305635203
Author:
Worrell, William A.
Publisher:
Cengage Learning,