The radioactive isotope Carbon-14 has a half-life of 5,730 years. Given 300 grams of C-14, what is the remaining mass (in grams) of the isotope after 250 years have elapsed? A) 291.06g B) 8.94g C) 97.02g D) 202.98g
The radioactive isotope Carbon-14 has a half-life of 5,730 years. Given 300 grams of C-14, what is the remaining mass (in grams) of the isotope after 250 years have elapsed? A) 291.06g B) 8.94g C) 97.02g D) 202.98g
Sustainable Energy
2nd Edition
ISBN:9781337551663
Author:DUNLAP, Richard A.
Publisher:DUNLAP, Richard A.
Chapter6: Energy From Nuclear Fission
Section: Chapter Questions
Problem 19P
Related questions
Question
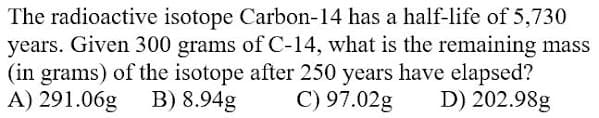
Transcribed Image Text:The radioactive isotope Carbon-14 has a half-life of 5,730
years. Given 300 grams of C-14, what is the remaining mass
(in grams) of the isotope after 250 years have elapsed?
A) 291.06g
B) 8.94g
C) 97.02g
D) 202.98g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, civil-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

