The table below shows the melting point, specific heat, and heat of fusion of different solids. Suppose the same mass of these solids are at their respective melting point temperatures, which solid requires most amount of heat to melt? Substance Melting Point Heat of Fusion Specific Heat 0.50 cal/gC 79.71 cal/g 5.85 cal/g Ice 0°C 327°C 0.033 cal/gC° 0.056 cal/gC° 0.030 cal/gC Lead 21.07 cal/g 15.39 cal/g 31.98 cal/g Silver 961°C Gold 1063°C Copper 1083°C 0.093 cal/gC° O lead O ice O gold O silver
The table below shows the melting point, specific heat, and heat of fusion of different solids. Suppose the same mass of these solids are at their respective melting point temperatures, which solid requires most amount of heat to melt? Substance Melting Point Heat of Fusion Specific Heat 0.50 cal/gC 79.71 cal/g 5.85 cal/g Ice 0°C 327°C 0.033 cal/gC° 0.056 cal/gC° 0.030 cal/gC Lead 21.07 cal/g 15.39 cal/g 31.98 cal/g Silver 961°C Gold 1063°C Copper 1083°C 0.093 cal/gC° O lead O ice O gold O silver
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter5: Analysis Of Convection Heat Transfer
Section: Chapter Questions
Problem 5.2P: 5.2 Evaluate the Prandtl number from the following data: , .
Related questions
Question
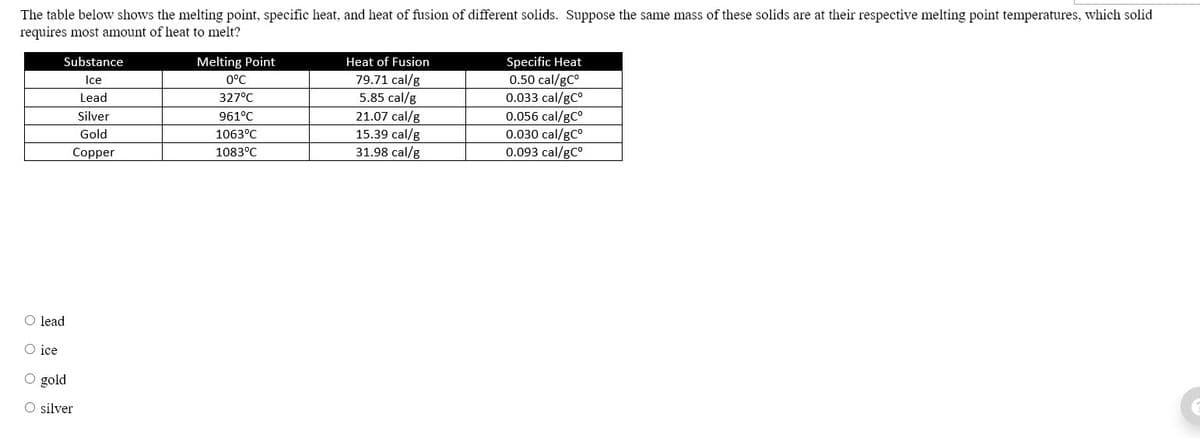
Transcribed Image Text:The table below shows the melting point, specific heat, and heat of fusion of different solids. Suppose the same mass of these solids are at their respective melting point temperatures, which solid
requires most amount of heat to melt?
Substance
Melting Point
Heat of Fusion
Specific Heat
79.71 cal/g
5.85 cal/g
21.07 cal/g
15.39 cal/g
31.98 cal/g
0.50 cal/gC°
0.033 cal/gC°
0.056 cal/gC°
0.030 cal/gCo
0.093 cal/gC°
Ice
0°C
Lead
327°C
Silver
961°C
Gold
1063°C
Copper
1083°C
O lead
ice
O gold
O silver
O O O O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning