The wavefunction of an hydrogenic atom in its ground state is: 3/2 1 Φο ✓ (1) *-* where Z is the atomic number and a the bohr radius. 1) Give an expression of ao as a function of the reduced mass. What is the reduced mass of a tritium atom (you can assume that the mass of a neutron to be the same as the mass of a proton)? What is the reduced mass of ³He+? How do they compare to the mass of an electron? 2) What is the wavefunction of a tritium atom in its ground state? What is the wavefunction of ³He+ in its ground state?
The wavefunction of an hydrogenic atom in its ground state is: 3/2 1 Φο ✓ (1) *-* where Z is the atomic number and a the bohr radius. 1) Give an expression of ao as a function of the reduced mass. What is the reduced mass of a tritium atom (you can assume that the mass of a neutron to be the same as the mass of a proton)? What is the reduced mass of ³He+? How do they compare to the mass of an electron? 2) What is the wavefunction of a tritium atom in its ground state? What is the wavefunction of ³He+ in its ground state?
Related questions
Question
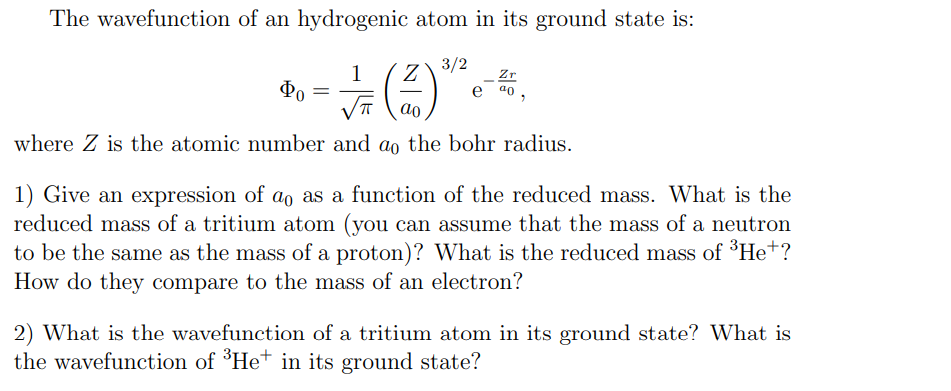
Transcribed Image Text:The wavefunction of an hydrogenic atom in its ground state is:
1
Z
3/2
Zr
Φο
a0
ao
where Z is the atomic number and do the bohr radius.
1) Give an expression of a。 as a function of the reduced mass. What is the
reduced mass of a tritium atom (you can assume that the mass of a neutron
to be the same as the mass of a proton)? What is the reduced mass of ³He+?
How do they compare to the mass of an electron?
2) What is the wavefunction of a tritium atom in its ground state? What is
the wavefunction of ³He+ in its ground state?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images
