Two moles of gas initially at a pressure of 2.00 atm and a volume of 0.300 L has internal energy equal to 91.0 J. In its final state, the gas is at a pressure of 1.50 atm and a volume of 0.800 L, and its internal energy equals 182 J. Three paths are plotted on a PV diagram, which has a horizontal axis labeled V (liters), and a vertical axis labeled P (atm). The green path starts at point I (0.300,2.00), extends vertically down to point A (0.300,1.50), then extends horizontally to point F (0.800,1.50). The blue path starts at point I (0.300,2.00), and extends down and to the right to end at point F (0.800,1.50). The orange path starts at point I (0.300,2.00), extends horizontally to the right to point B (0.800,2.00), then extends vertically down to end at point F (0.800,1.50). (a) For the paths IAF, IBF, and IF in the figure above, calculate the work done on the gas. WIAF = Your response differs from the correct answer by more than 10%. Double check your calculations. J WIBF = J WIF = J (b) For the paths IAF, IBF, and IF in the figure above, calculate the net energy transferred to the gas by heat in the process. QIAF = J QIBF = J QIF = J
Two moles of gas initially at a pressure of 2.00 atm and a volume of 0.300 L has internal energy equal to 91.0 J. In its final state, the gas is at a pressure of 1.50 atm and a volume of 0.800 L, and its internal energy equals 182 J. Three paths are plotted on a PV diagram, which has a horizontal axis labeled V (liters), and a vertical axis labeled P (atm). The green path starts at point I (0.300,2.00), extends vertically down to point A (0.300,1.50), then extends horizontally to point F (0.800,1.50). The blue path starts at point I (0.300,2.00), and extends down and to the right to end at point F (0.800,1.50). The orange path starts at point I (0.300,2.00), extends horizontally to the right to point B (0.800,2.00), then extends vertically down to end at point F (0.800,1.50). (a) For the paths IAF, IBF, and IF in the figure above, calculate the work done on the gas. WIAF = Your response differs from the correct answer by more than 10%. Double check your calculations. J WIBF = J WIF = J (b) For the paths IAF, IBF, and IF in the figure above, calculate the net energy transferred to the gas by heat in the process. QIAF = J QIBF = J QIF = J
Related questions
Question
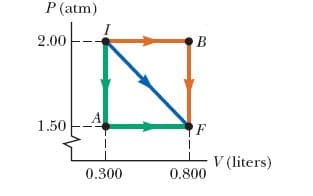
Two moles of gas initially at a pressure of 2.00 atm and a volume of 0.300 L has internal energy equal to 91.0 J. In its final state, the gas is at a pressure of 1.50 atm and a volume of 0.800 L, and its internal energy equals 182 J.
Three paths are plotted on a PV diagram, which has a horizontal axis labeled V (liters), and a vertical axis labeled P (atm).
- The green path starts at point I (0.300,2.00), extends vertically down to point A (0.300,1.50), then extends horizontally to point F (0.800,1.50).
- The blue path starts at point I (0.300,2.00), and extends down and to the right to end at point F (0.800,1.50).
- The orange path starts at point I (0.300,2.00), extends horizontally to the right to point B (0.800,2.00), then extends vertically down to end at point F (0.800,1.50).
(a) For the paths IAF, IBF, and IF in the figure above, calculate the work done on the gas.
(b) For the paths IAF, IBF, and IF in the figure above, calculate the net energy transferred to the gas by heat in the process.
| WIAF | = | Your response differs from the correct answer by more than 10%. Double check your calculations. J |
| WIBF | = | J |
| WIF | = | J |
(b) For the paths IAF, IBF, and IF in the figure above, calculate the net energy transferred to the gas by heat in the process.
| QIAF | = | J |
| QIBF | = | J |
| QIF | = | J |
Transcribed Image Text:P (atm)
2.00
В
1.50
F
V (liters)
0.300
0.800
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps
