Use the talble to detemine the energy in evof the photon emitted when thee bectran. Jumps down from the n=4 orbit of a tasy hydrogen atom Allowed the values of the Hydrogen electrons Raclius and Energy fr lows values ofn I En 10.53nm -13.60ev. 20,212nm | 3.40eV 3 0,477nm M0.848nm -0.8ser -l05lev |
Use the talble to detemine the energy in evof the photon emitted when thee bectran. Jumps down from the n=4 orbit of a tasy hydrogen atom Allowed the values of the Hydrogen electrons Raclius and Energy fr lows values ofn I En 10.53nm -13.60ev. 20,212nm | 3.40eV 3 0,477nm M0.848nm -0.8ser -l05lev |
Related questions
Question
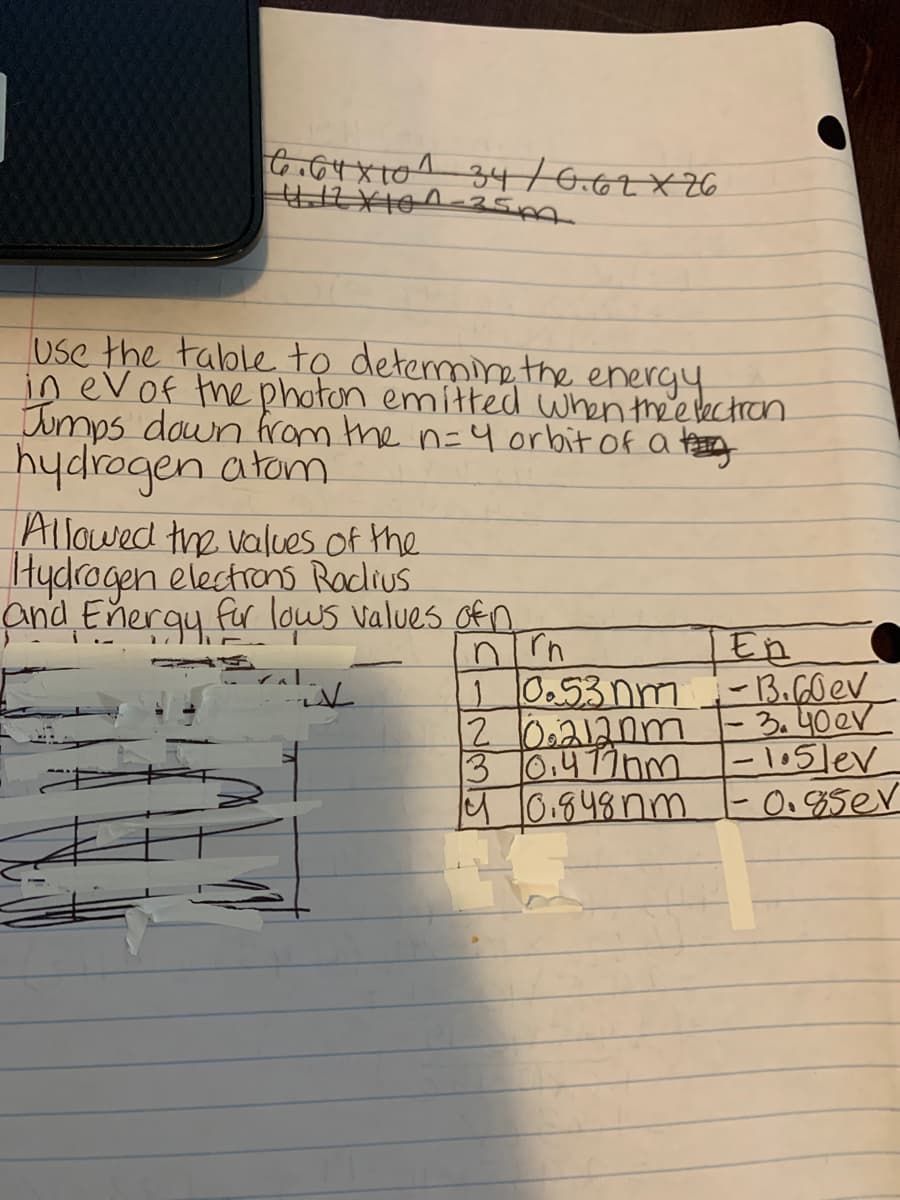
Transcribed Image Text:Use the talble to detemine the energy
in evof the photon emitted when thee bectran.
Jumps down from the n=4 orbit of a tasy
hydrogen atom
Allowed the values of the
Hydrogen electrons Raclius
and Energy fr lows values ofn
I En
10.53nm -13.60ev.
20,212nm |
3.40eV
3 0,477nm
M0.848nm -0.8ser
-l05lev
|
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
