Water standing in the open at 33.4°C evaporates because of the escape of some of the surface molecules. The heat of vaporization (530 cal/g) is approximately equal to En, where t is the average energy of the escaping molecules and n is the number of molecules per gram. (a) Find c. (b) What is the ratio of c to the average kinetic energy of H₂O molecules, assuming the latter is related to temperature in the same way as it is for gases?
Water standing in the open at 33.4°C evaporates because of the escape of some of the surface molecules. The heat of vaporization (530 cal/g) is approximately equal to En, where t is the average energy of the escaping molecules and n is the number of molecules per gram. (a) Find c. (b) What is the ratio of c to the average kinetic energy of H₂O molecules, assuming the latter is related to temperature in the same way as it is for gases?
Related questions
Question
Please no hand writing solution for other wise down vote
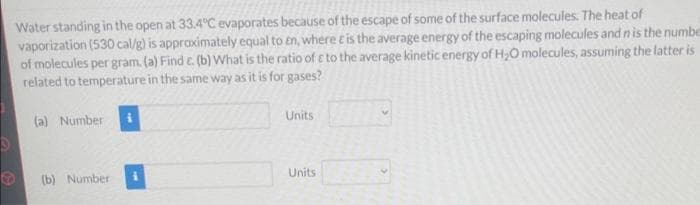
Transcribed Image Text:Water standing in the open at 33.4°C evaporates because of the escape of some of the surface molecules. The heat of
vaporization (530 cal/g) is approximately equal to En, where t is the average energy of the escaping molecules and n is the numbe
of molecules per gram. (a) Find E. (b) What is the ratio of c to the average kinetic energy of H₂O molecules, assuming the latter is
related to temperature in the same way as it is for gases?
(a) Number
(b) Number
Units
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps
