What generalization can one make concerning the relationship between two properties of elements?
What generalization can one make concerning the relationship between two properties of elements?
Database System Concepts
7th Edition
ISBN:9780078022159
Author:Abraham Silberschatz Professor, Henry F. Korth, S. Sudarshan
Publisher:Abraham Silberschatz Professor, Henry F. Korth, S. Sudarshan
Chapter1: Introduction
Section: Chapter Questions
Problem 1PE
Related questions
Question
I need help

Transcribed Image Text:What generalization can one make concerning the relationship between
two properties of elements? *
1p
O A. As the atomic radius decreases, the electronegativity decreases.
O B. As the atomic radius decreases, the electronegativity increases.
O C. All metals have higher electronegativity values than non-metals.
O D. The atomic radius of F is larger than that of N.
Which of the following is true regarding the comparative electronegativity 1 point
of fluorine (F) and lithium (LI)?*
F. The electronegativity of F is greater than Li because F has fewer electrons in its
outer shell.
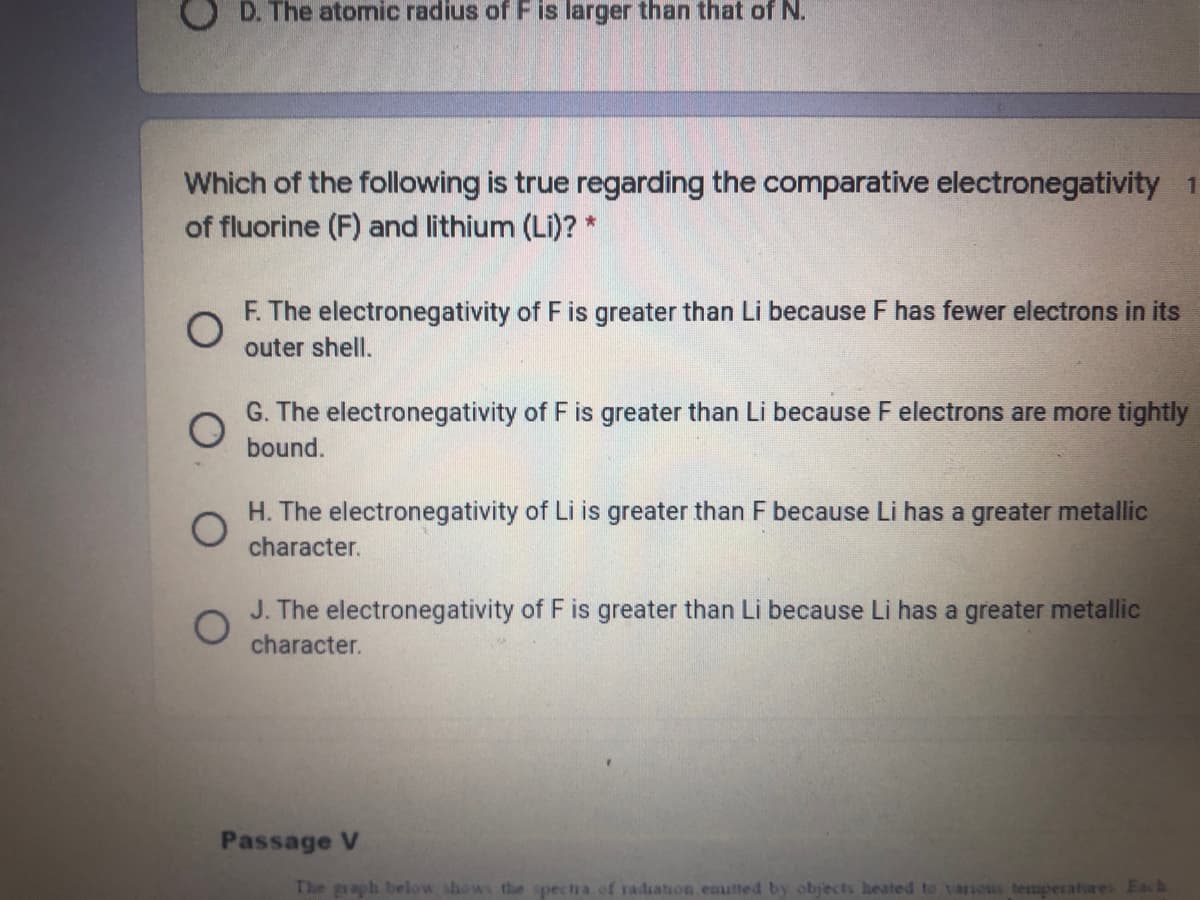
Transcribed Image Text:D. The atomic radius of Fis larger than that of N.
Which of the following is true regarding the comparative electronegativity 1
of fluorine (F) and lithium (Li)? *
F. The electronegativity of F is greater than Li because F has fewer electrons in its
outer shell.
G. The electronegativity of F is greater than Li because F electrons are more tightly
bound.
H. The electronegativity of Li is greater than F because Li has a greater metallic
character.
J. The electronegativity of F is greater than Li because Li has a greater metallic
character.
Passage V
The graph below shows the spectra of radiation emutted by objects heated to v anous temperatures Each
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, computer-science and related others by exploring similar questions and additional content below.Recommended textbooks for you

Database System Concepts
Computer Science
ISBN:
9780078022159
Author:
Abraham Silberschatz Professor, Henry F. Korth, S. Sudarshan
Publisher:
McGraw-Hill Education

Starting Out with Python (4th Edition)
Computer Science
ISBN:
9780134444321
Author:
Tony Gaddis
Publisher:
PEARSON

Digital Fundamentals (11th Edition)
Computer Science
ISBN:
9780132737968
Author:
Thomas L. Floyd
Publisher:
PEARSON

Database System Concepts
Computer Science
ISBN:
9780078022159
Author:
Abraham Silberschatz Professor, Henry F. Korth, S. Sudarshan
Publisher:
McGraw-Hill Education

Starting Out with Python (4th Edition)
Computer Science
ISBN:
9780134444321
Author:
Tony Gaddis
Publisher:
PEARSON

Digital Fundamentals (11th Edition)
Computer Science
ISBN:
9780132737968
Author:
Thomas L. Floyd
Publisher:
PEARSON

C How to Program (8th Edition)
Computer Science
ISBN:
9780133976892
Author:
Paul J. Deitel, Harvey Deitel
Publisher:
PEARSON

Database Systems: Design, Implementation, & Manag…
Computer Science
ISBN:
9781337627900
Author:
Carlos Coronel, Steven Morris
Publisher:
Cengage Learning

Programmable Logic Controllers
Computer Science
ISBN:
9780073373843
Author:
Frank D. Petruzella
Publisher:
McGraw-Hill Education