When a metal was exposed to photons at a frequency of 1.25 × 10¹5 s¯¹, electrons were emitted with a maximum kinetic energy of 3.10 x 10-1⁹ J. Calculate the work function, P, of this metal. = J/photon What is the maximum number of electrons that could be ejected from this metal by a burst of photons (at some other frequency) with a total energy of 1.48 x 10-7 J? number of electrons:
When a metal was exposed to photons at a frequency of 1.25 × 10¹5 s¯¹, electrons were emitted with a maximum kinetic energy of 3.10 x 10-1⁹ J. Calculate the work function, P, of this metal. = J/photon What is the maximum number of electrons that could be ejected from this metal by a burst of photons (at some other frequency) with a total energy of 1.48 x 10-7 J? number of electrons:
Related questions
Question
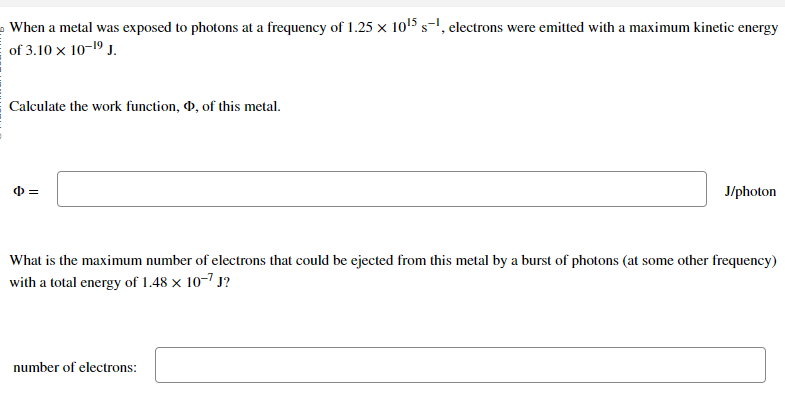
Transcribed Image Text:When a metal was exposed to photons at a frequency of 1.25 x 10¹5 s-¹, electrons were emitted with a maximum kinetic energy
of 3.10 × 10-1⁹ J.
Calculate the work function, , of this metal.
=
J/photon
What is the maximum number of electrons that could be ejected from this metal by a burst of photons (at some other frequency)
with a total energy of 1.48 x 10-7 J?
number of electrons:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 10 images
