When heated, oxygen reacts with copper to form copper oxide. + • + • oxygen molecule copper atoms copper oxide molecules Key: Oxygen atom Copper atom If this reaction occurs in a sealed container, will the mass of the container and everything in it increase, decrease, or stay the same and why? The mass will stay the same because the number of each kind of atom stays O A the same. The mass will decrease because two substances combine to form one B substance. The mass will increase because a new kind of molecule is formed.
When heated, oxygen reacts with copper to form copper oxide. + • + • oxygen molecule copper atoms copper oxide molecules Key: Oxygen atom Copper atom If this reaction occurs in a sealed container, will the mass of the container and everything in it increase, decrease, or stay the same and why? The mass will stay the same because the number of each kind of atom stays O A the same. The mass will decrease because two substances combine to form one B substance. The mass will increase because a new kind of molecule is formed.
Related questions
Question
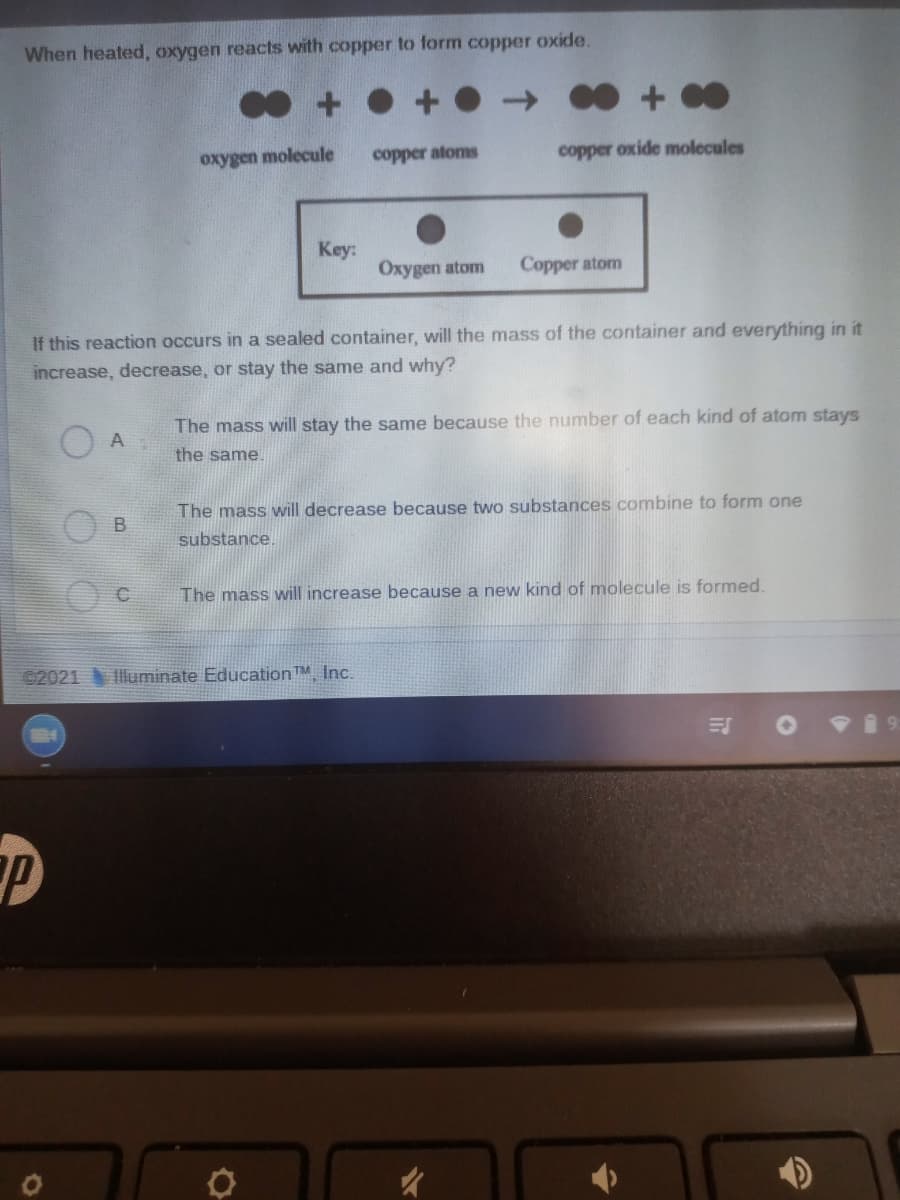
Transcribed Image Text:When heated, oxygen reacts with copper to form copper oxide.
oxygen molecule
copper atoms
copper oxide molecules
Key:
Oxygen atom
Copper atom
If this reaction occurs in a sealed container, will the mass of the container and everything in it
increase, decrease, or stay the same and why?
The mass will stay the same because the number of each kind of atom stays
the same.
The mass will decrease because two substances combine to form one
substance
The mass will increase because a new kind of molecule is formed.
©2021 lluminate EducationTM Inc.

Transcribed Image Text:More information is needed to tell if the mass will change.
©2021
Illuminate Education TM, Inc.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
