your answers separated by Uz avg, Vy avg" 0,0 m/s The molecules in a six-particle gas have velocities 7 = (20i – 30j) m/s iz = (50i + 90j) m/s i = (-90i + 20j) m/s Submit Previous Answers v Correct v4 = 30i m/s is = (40i – 40j) m/s 7 = (-50i – 40j) m/s Part B Calculate avg Express your answer to two significant figures and include the appropriate units. Vavg = 0 Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Part C Çalculate trm Express your answer to two significant figures and include the appropriate units. Urm- 69
your answers separated by Uz avg, Vy avg" 0,0 m/s The molecules in a six-particle gas have velocities 7 = (20i – 30j) m/s iz = (50i + 90j) m/s i = (-90i + 20j) m/s Submit Previous Answers v Correct v4 = 30i m/s is = (40i – 40j) m/s 7 = (-50i – 40j) m/s Part B Calculate avg Express your answer to two significant figures and include the appropriate units. Vavg = 0 Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Part C Çalculate trm Express your answer to two significant figures and include the appropriate units. Urm- 69
Related questions
Question
Transcribed Image Text:Const
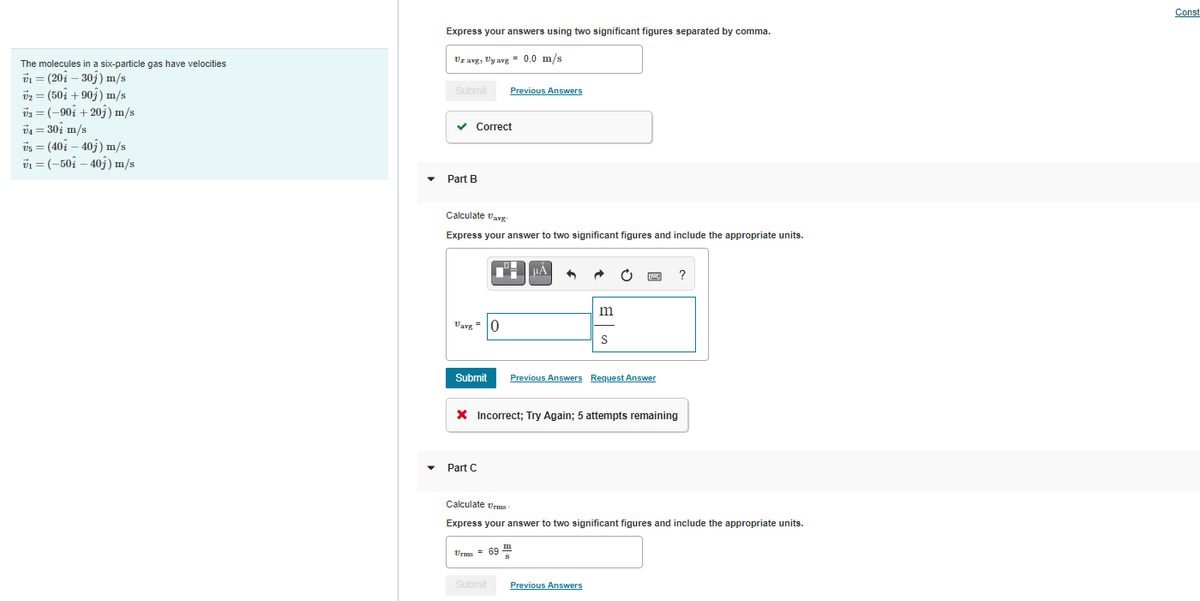
Express your answers using two significant figures separated by comma.
Vz avg, Vy avg = 0,0 m/s
The molecules in a six-particle gas have velocities
v1 = (20i – 30j) m/s
v2 = (50i + 907) m/s
Submit
Previous Answers
v3 = (-90i + 20j) m/s
v4 = 30i m/s
vs = (40i – 40j) m/s
v1 = (-50; – 403) m/s
v Correct
Part B
Calculate vavg
Express your answer to two significant figures and include the appropriate units.
HA
m
Vavg = 0
S
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
Part C
Calculate vrms
Express your answer to two significant figures and include the appropriate units.
Vrms = 69 "
Submit
Previous Answers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
