-Z/Zo 3 The density of air as a function of altitude above sea level is modeled as p(z) = poe` where po = 1.2 kg/m³, and zo = 16, 855 m. A spherical balloon with radius r = 0.2 m is filled with Helium gas (MH₂ = 4 g/mol) to STP (P = 1 atm, T = 273 K). What is the maximum height the balloon can float up to assuming constant pressure and temperature within the balloon? Assume that the density of air is constant across the volume of the balloon for a specific height, and that the balloon's rubber has negligible mo
-Z/Zo 3 The density of air as a function of altitude above sea level is modeled as p(z) = poe` where po = 1.2 kg/m³, and zo = 16, 855 m. A spherical balloon with radius r = 0.2 m is filled with Helium gas (MH₂ = 4 g/mol) to STP (P = 1 atm, T = 273 K). What is the maximum height the balloon can float up to assuming constant pressure and temperature within the balloon? Assume that the density of air is constant across the volume of the balloon for a specific height, and that the balloon's rubber has negligible mo
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter5: Analysis Of Convection Heat Transfer
Section: Chapter Questions
Problem 5.5P: Evaluate the dimensionless groups hcD/k,UD/, and cp/k for water, n-butyl alcohol, mercury, hydrogen,...
Related questions
Question
**see image for question**

Transcribed Image Text:The density of air as a function of altitude above sea level is modeled as p(z) = Pᵒe¯z/zo, where Po = 1.2
kg/m³, and Zo 16, 855 m. A spherical balloon with radius r = 0.2 m is filled with Helium gas (MHe = 4
g/mol) to STP (P = 1 atm, T = 273 K). What is the maximum height the balloon can float up to
assuming constant pressure and temperature within the balloon? Assume that the density of air is
constant across the volume of the balloon for a specific height, and that the balloon's rubber has
negligible mass.
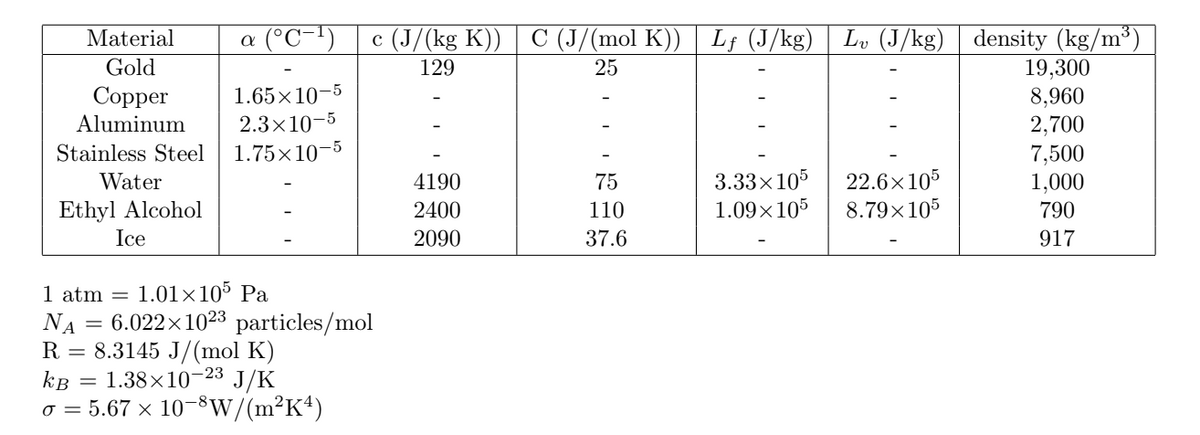
Transcribed Image Text:a (°℃−¹)
Material
Gold
Copper
Aluminum
1.65×10-5
2.3x10-5
Stainless Steel 1.75×10-5
Water
Ethyl Alcohol
Ice
1 atm = 1.01×105 Pa
NA = 6.022×102³ particles/mol
R = 8.3145 J/(mol K)
kB = 1.38×10-23 J/K
o = 5.67 × 10-8W/(m²K4)
c (J/(kg K)) C (J/(mol K)) | Lf (J/kg) Lv (J/kg) density (kg/m³)
129
25
19,300
8,960
2,700
7,500
4190
75
1,000
3.33×105 22.6×105
1.09×105 8.79×105
2400
110
790
2090
37.6
917
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning