. Use data from appendix B (attached phhoto) to obtain the equilibrium constant Kp for each reaction at 298.15 K. A. 2 HCl(g) ---> H2 (g) + Cl2 (g) B. N2(g) + O2(g) ---> 2 NO(g)
. Use data from appendix B (attached phhoto) to obtain the equilibrium constant Kp for each reaction at 298.15 K. A. 2 HCl(g) ---> H2 (g) + Cl2 (g) B. N2(g) + O2(g) ---> 2 NO(g)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section: Chapter Questions
Problem 16.ECP: Consider planet Earth as a thermodynamic system. Is Earth thermodynamically or kinetically stable?...
Related questions
Question
100%
24. Use data from appendix B (attached phhoto) to obtain the equilibrium constant Kp for each reaction at 298.15 K.
A. 2 HCl(g) ---> H2 (g) + Cl2 (g)
B. N2(g) + O2(g) ---> 2 NO(g)
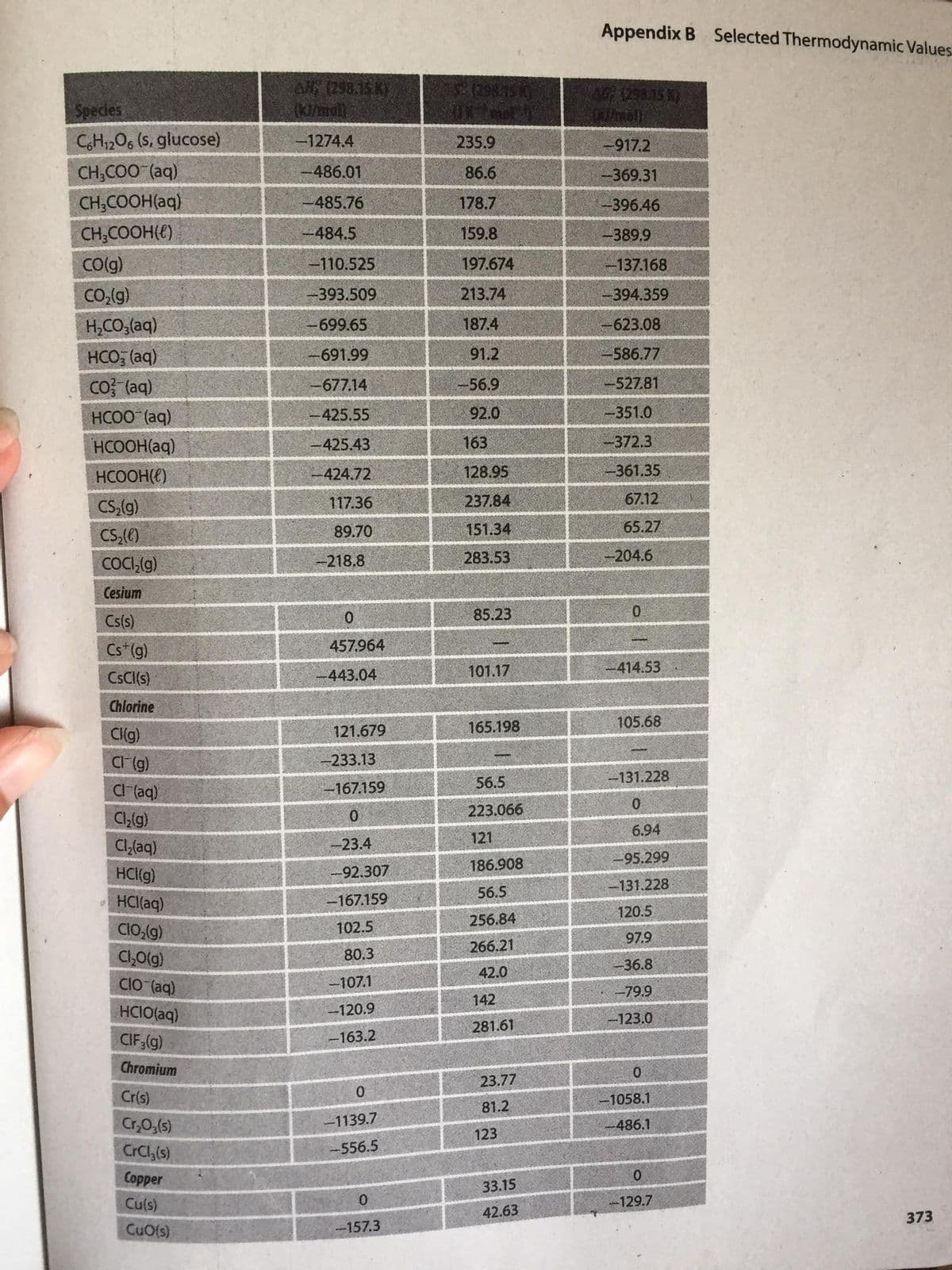
Transcribed Image Text:Appendix B Selected Thermodynamic Values
A 1298.15 K)
(K/mall.
Speces
CH12O6 (S, glucose)
1274.4
235.9
-917.2
CH,COO (aq)
486.01
86.6
-369.31
CH;COOH(aq)
485.76
178.7
--396.46
CH;COOH(e)
484.5
159.8
-389.9
Co(g)
-110.525
197.674
-137.168
CO,(g)
-393.509
213.74
394.359
H,CO,(aq)
-699.65
187.4
-623.08
691.99
91.2
586.77
HCO, (aq)
Co? (aq)
677.14
56.9
-527.81
HCOO (aq)
425.55
92.0
-351.0
HCOOH(aq)
425.43
163
--372.3
HCOOH(E)
424.72
128.95
-361.35
CS,(g)
117.36
237.84
67.12
CS;()
89.70
151.34
65.27
COC,(g)
-218.8
283.53
-204.6
Cesium
85.23
0.
Cs(s)
Cs* (g)
457.964
101.17
414.53
CsC(s)
-443.04
Chlorine
165.198
105.68
Cl(g)
121.679
Cl (g)
-233.13
56.5
-131.228
C (aq)
-167.159
Ch!g)
0.
223.066
6.94
121
Cl,(aq)
-23.4
-95.299
186.908
HCI(g)
-92.307
-131.228
56.5
HCl(aq)
-167.159
120.5
256.84
CIO,(g)
102.5
97.9
266.21
C1,0(g)
80.3
36.8
42.0
CIO (aq)
-107.1
-79.9
142
HCIO(aq)
-120.9
-123.0
281.61
CIF,(g)
-163.2
Chromium
23.77
Cr(s)
-1058.1
81.2
Cr,0,(s)
-1139.7
-486.1
123
CrCl,(s)
556.5
Сopper
0.
33.15
Cu(s)
0.
-129.7
42.63
373
CuO(s)
-157.3
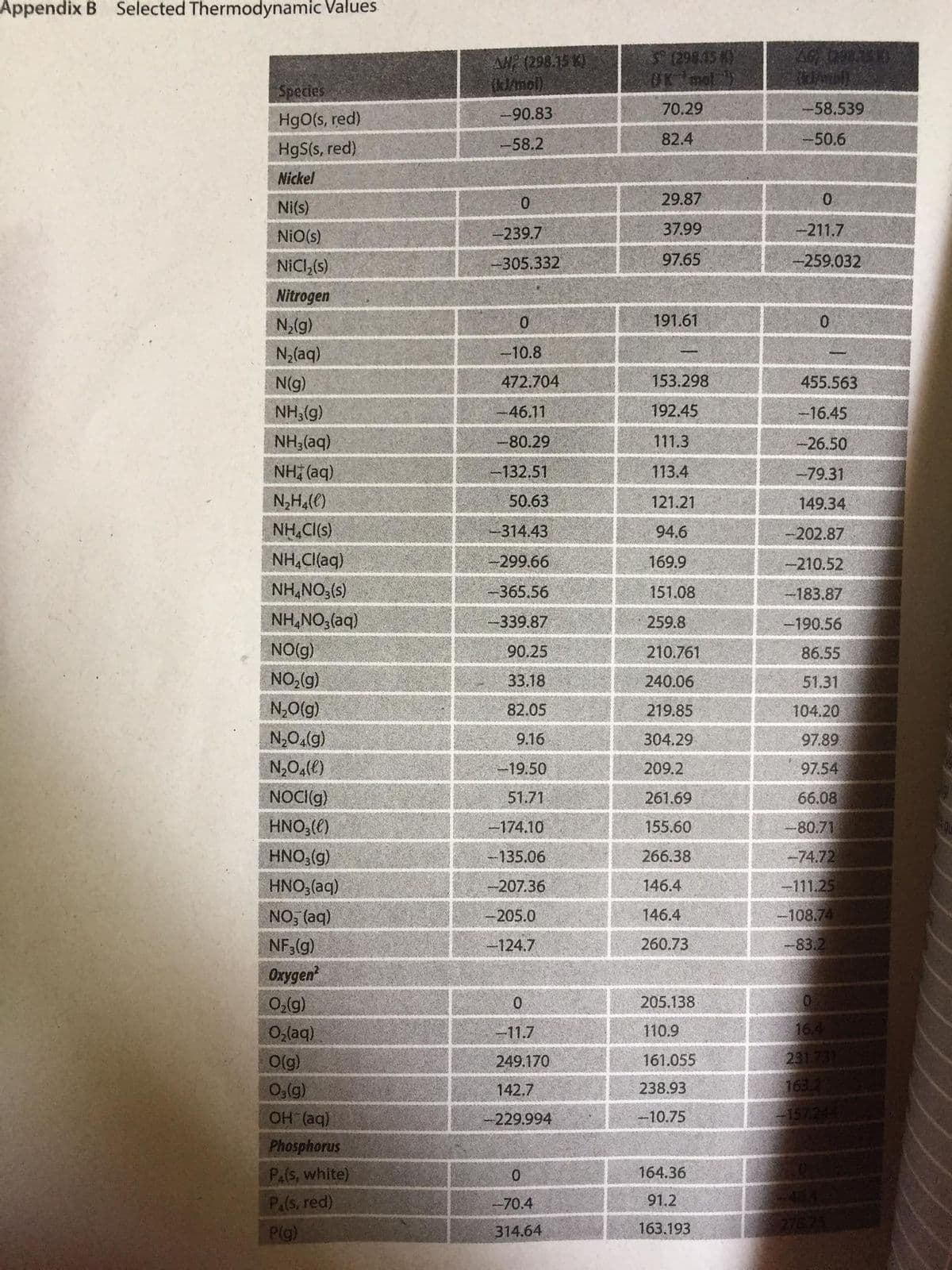
Transcribed Image Text:Appendix B Selected Thermodynamic Values
AG (29
AH (298.15 K)
(klmol)
S (298.45 8)
OK ol
Species
HgO(s, red)
-90.83
70.29
-58.539
82.4
50.6
HgS(s, red)
-58.2
Nickel
29.87
Ni(s)
NiO(s)
-239.7
37.99
-211.7
NICI,(s)
-305.332
97.65
-259.032
Nitrogen
N2(g)
191.61
0.
N,(aq)
-10.8
N(g)
472.704
153.298
455.563
NH3(g)
-46.11
192.45
-16.45
NH3(aq)
-80.29
111.3
-26.50
NH (aq)
-132.51
113.4
-79.31
N,H,()
50.63
121.21
149.34
NH,CI(s)
314.43
94.6
-202.87
NH,Cl(aq)
-299.66
169.9
-210.52
NH,NO3(s)
365.56
151.08
183.87
NH,NO,(aq)
339.87
259.8
-190.56
NO(g)
90.25
210.761
86.55
NO,(g)
33.18
240.06
51.31
N,0(g)
82.05
219.85
104.20
N,0,(g)
9.16
304.29
97.89
N,0,(e)
-19.50
209.2
97.54
NOCI(g)
51.71
51.69
66.
HNO,()
-174.10
155.60
-80.71
HNO,(g)
-135.06
266.38
-74.72
HNO;(aq)
-207.36
146.4
-111.25
NO, (aq)
205.0
146.4
-108.74
260.73
-83.2
NF3(g)
Oxygen
-124.7
0,(g)
0.
205.138
O,laq)
-11.7
110.9
O(g)
249.170
161.055
231.731
Og(g)
142,7
238.93
163
OH (aq)
229.994
-10.75
157.244
Phosphorus
Pa(s, white)
0.
164.36
Pals, red)
-70.4
91.2
Plg)
163.193
7675
314.64
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning