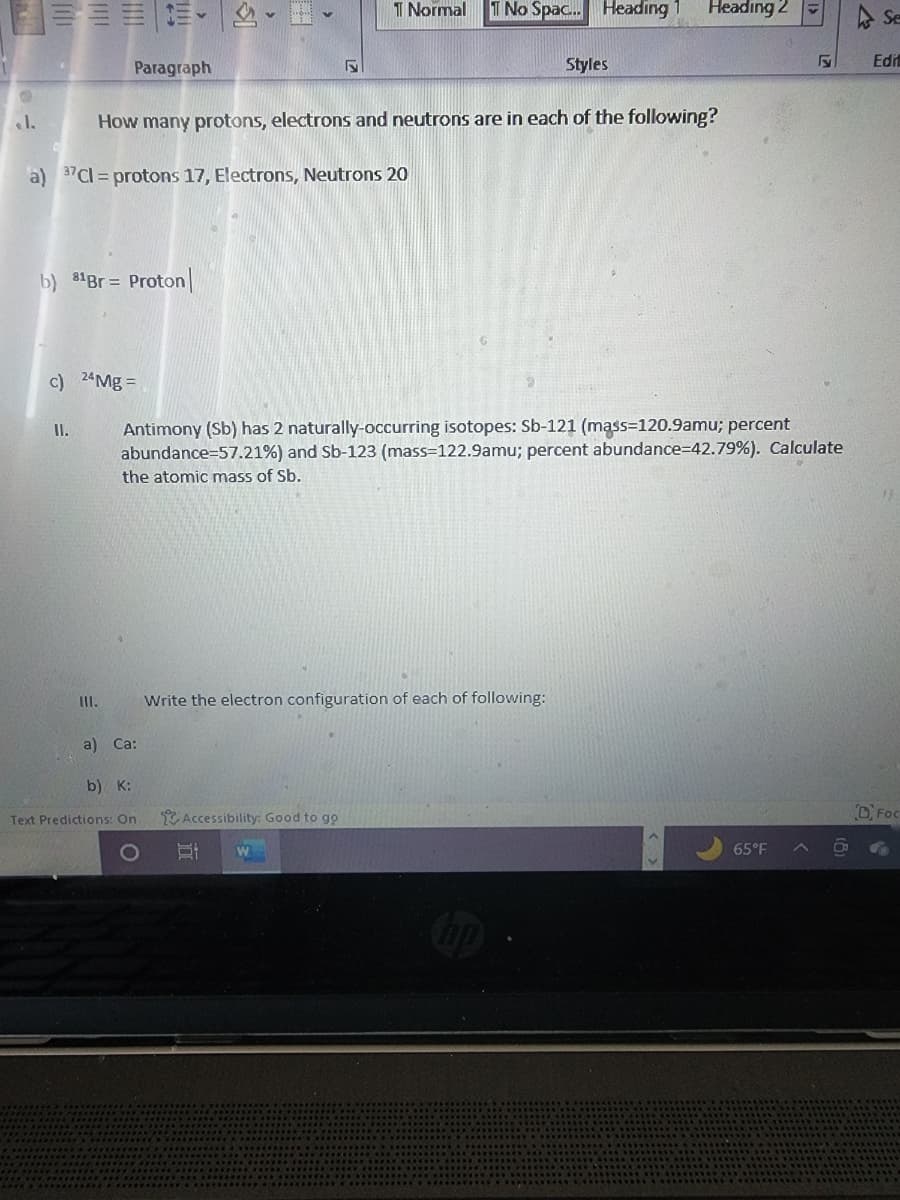
... How many protons, electrons and neutrons are in each of the following? a) 37Cl = protons 17, Electrons, Neutrons 20 b) 8¹Br = Proton| c) 24Mg = II. III. Antimony (Sb) has 2 naturally-occurring isotopes: Sb-121 (mass=120.9amu; percent abundance-57.21%) and Sb-123 (mass=122.9amu; percent abundance-42.79%). Calculate the atomic mass of Sb. a) Ca: b) K: Write the electron configuration of each of following:
... How many protons, electrons and neutrons are in each of the following? a) 37Cl = protons 17, Electrons, Neutrons 20 b) 8¹Br = Proton| c) 24Mg = II. III. Antimony (Sb) has 2 naturally-occurring isotopes: Sb-121 (mass=120.9amu; percent abundance-57.21%) and Sb-123 (mass=122.9amu; percent abundance-42.79%). Calculate the atomic mass of Sb. a) Ca: b) K: Write the electron configuration of each of following:
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 23E: Average atomic masses listed by JUPAC are based on a study of experimental results. Bromine has two...
Related questions
Question
Attached chemistry questions
Transcribed Image Text:O
I.
c)
Paragraph
b) 81Br = Proton|
II.
a) 37Cl = protons 17, Electrons, Neutrons 20
III.
24 Mg =
How many protons, electrons and neutrons are in each of the following?
a) Ca:
b) K:
Text Predictions: On
5
O
T Normal 1 No Spac... Heading 1
Antimony (Sb) has 2 naturally-occurring isotopes: Sb-121 (mass=120.9amu; percent
abundance-57.21%) and Sb-123 (mass=122.9amu; percent abundance-42.79%). Calculate
the atomic mass of Sb.
Accessibility: Good to go
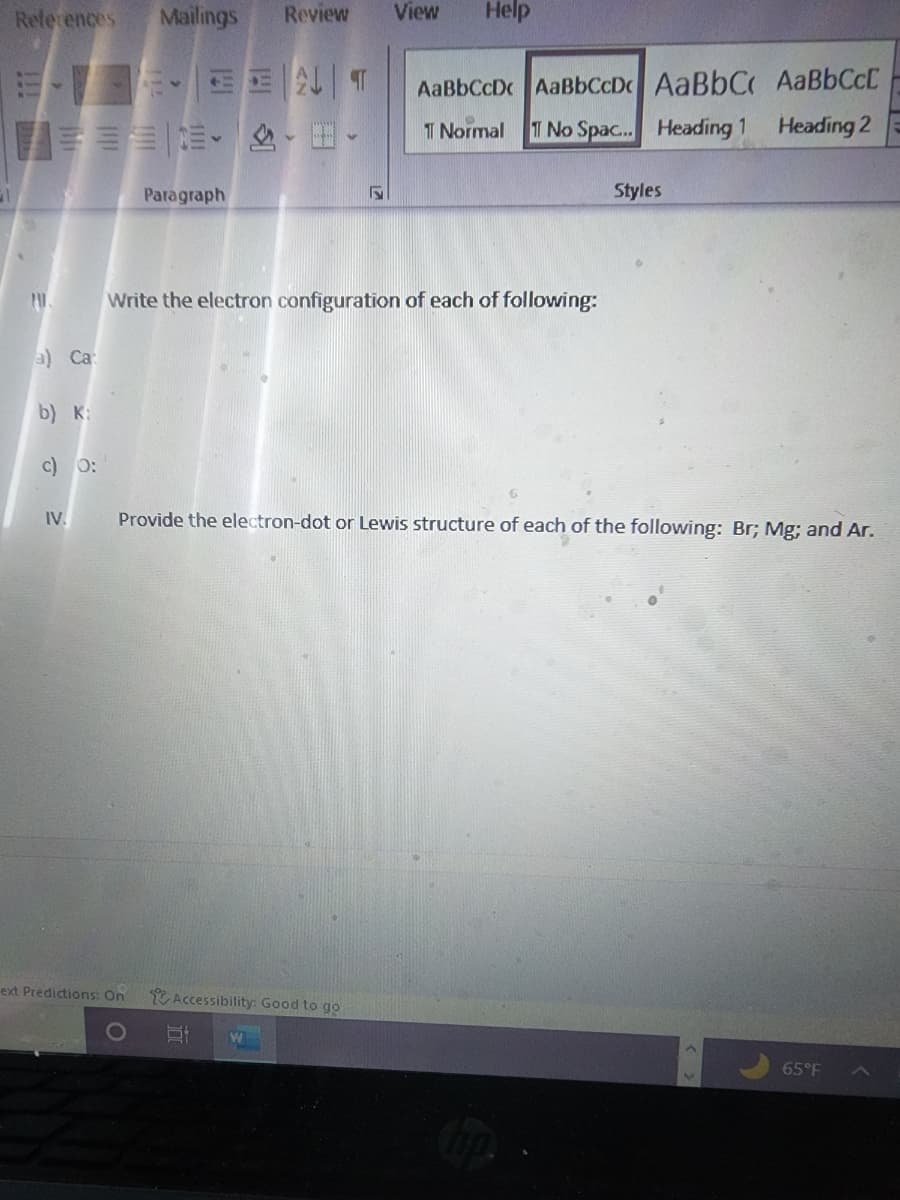
Write the electron configuration of each of following:
F
Ri
Styles
Heading 2
65°F
O
Se
Edit
Foc
Transcribed Image Text:References Mailings Review
EAT
PI.
Paragraph
ext Predictions: On
Write the electron configuration of each of following:
O
View Help
a) Ca
b) K:
c) 0:
IV. Provide the electron-dot or Lewis structure of each of the following: Br; Mg; and Ar.
Accessibility: Good to go
AaBbCcDc | AaBbCcD. AaBbC AaBbCcE
T Normal
T No Spac...
Heading 1
Heading 2
B
Styles
65°F
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning