005) What is the effect on the mass number of the parent nuclide when each of the following occurs? a) Alpha particle decay b) Beta particle decay c) Gamma ray emission
005) What is the effect on the mass number of the parent nuclide when each of the following occurs? a) Alpha particle decay b) Beta particle decay c) Gamma ray emission
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter18: The Nucleus: A Chemist's View
Section: Chapter Questions
Problem 15E
Related questions
Question
what is the effect on the mass number of the parent nuclide when each of the following occurs
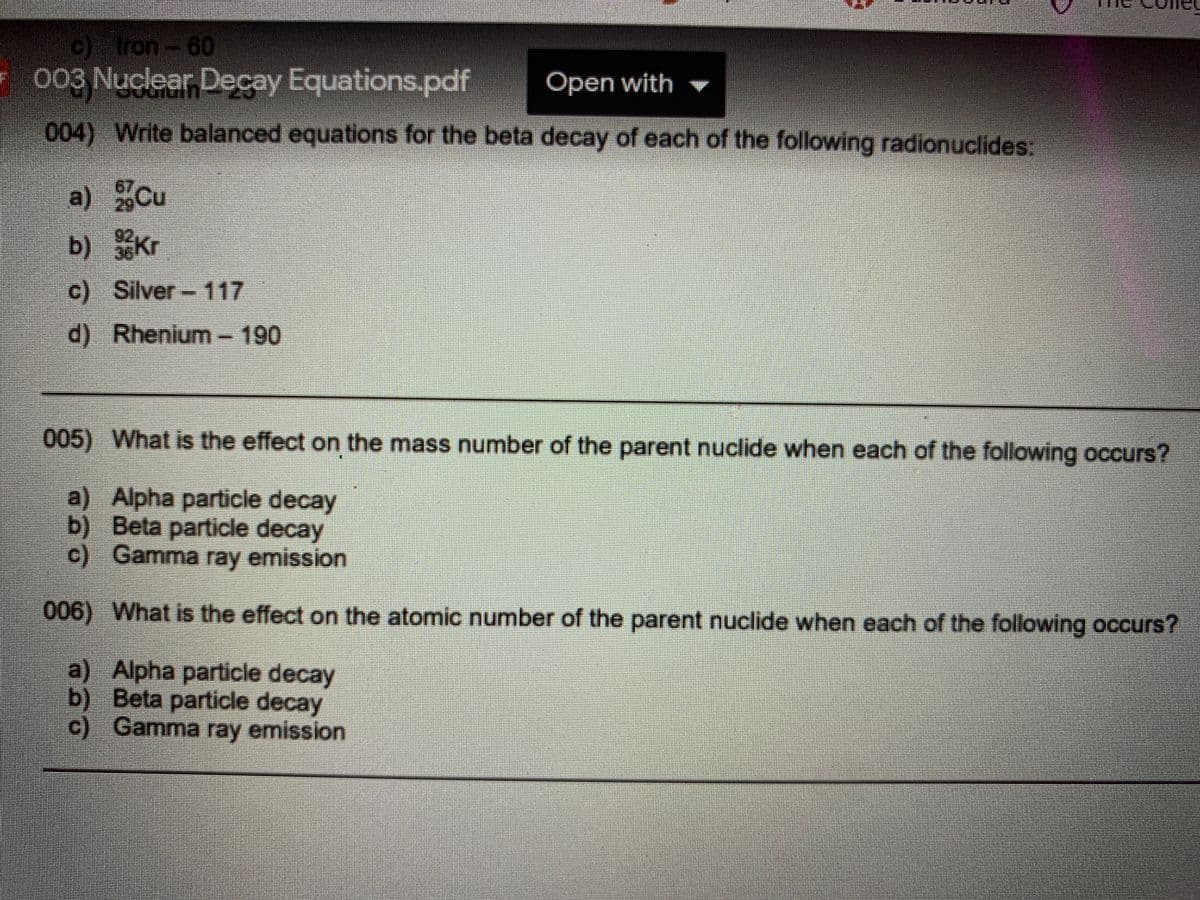
Transcribed Image Text:) Iron-60
003 Nuclear Decay Equations.pdf
Open with
004) Write balanced equations for the beta decay of each of the following radionuclides:
a) Cu
b) Kr
c) Silver- 117
d) Rhenium-190
005) What is the effect on the mass number of the parent nuclide when each of the following occurs?
a) Alpha particle decay
b) Beta particle decay
c) Gamma emission
ray
006) What is the effect on the atomic number of the parent nuclide when each of the following occurs?
a) Alpha particle decay
b) Beta particle decay
c) Gamma ray emission
Expert Solution
Step 1
a) In case of alpha decay ,emission of a helium nuclei takes place i.e. 2He4 hence we can see that mass number of a parent nucleus get decreased by a value of 4 unit.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning