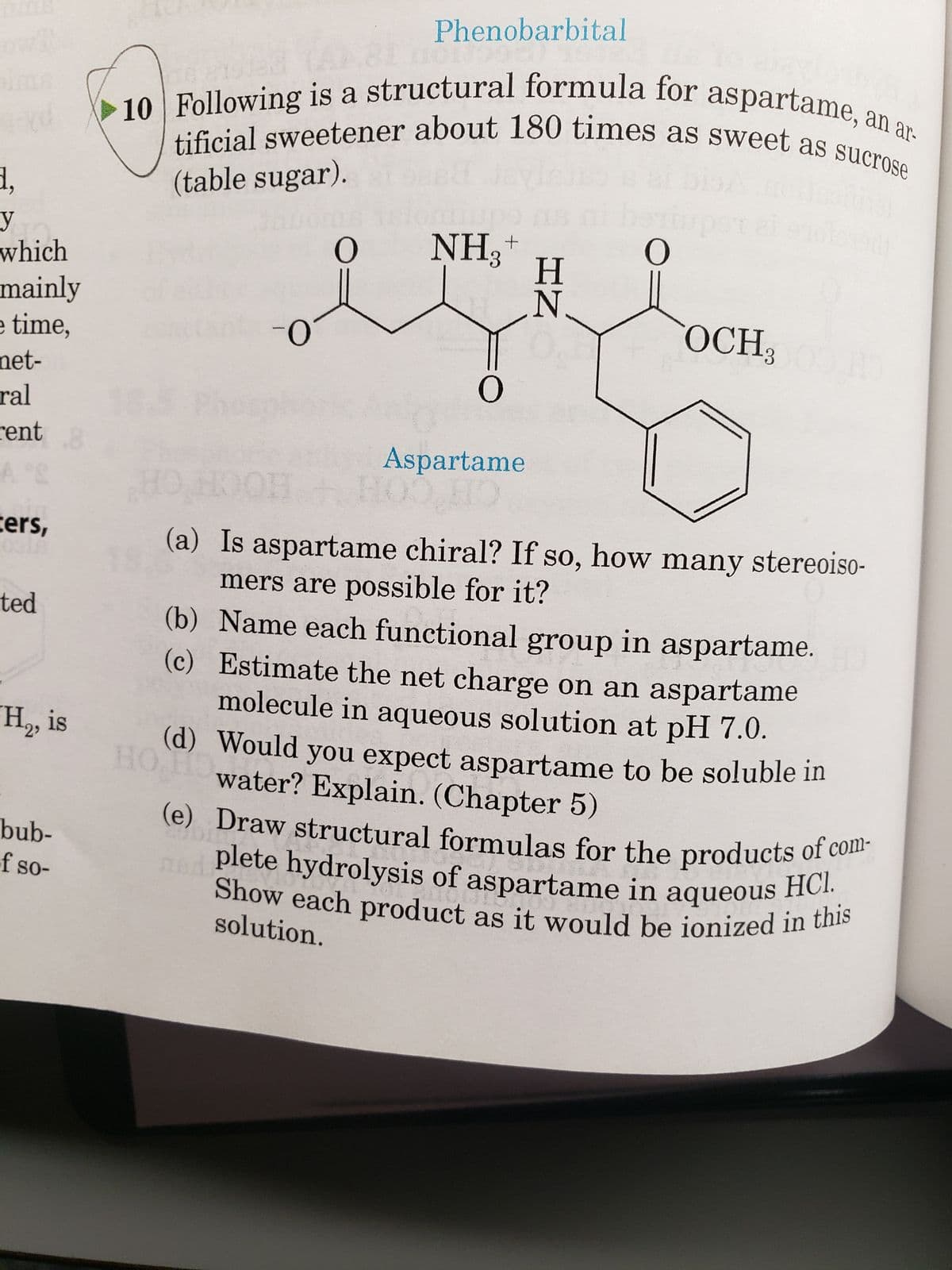
1 ly e, S 10 Following is a structural formula for aspartame, an ar- tificial sweetener about 180 times as sweet as sucrose (table sugar). 0 O (b) (c) NH3 O Aspartame H N O OCH300 H (a) Is aspartame chiral? If so, how many stereoiso- mers are possible for it? Name each functional group in aspartame. Estimate the net charge on an aspartame molecule in aqueous solution at pH 7.0. (d) Would
1 ly e, S 10 Following is a structural formula for aspartame, an ar- tificial sweetener about 180 times as sweet as sucrose (table sugar). 0 O (b) (c) NH3 O Aspartame H N O OCH300 H (a) Is aspartame chiral? If so, how many stereoiso- mers are possible for it? Name each functional group in aspartame. Estimate the net charge on an aspartame molecule in aqueous solution at pH 7.0. (d) Would
Chapter25: Biomolecules: Carbohydrates
Section25.SE: Something Extra
Problem 69AP
Related questions
Question
Transcribed Image Text:d.
y
which
mainly
e time,
net-
ral
rent .8
ters,
ted
H₂, is
bub-
of so-
nolad
(table sugar).
10 Following is a structural formula for aspartame, an ar-
tificial sweetener about 180 times as sweet as sucrose
HO
-O
(d)
O
Phenobarbital
NH₂+
O
Aspartame
Η
HN
요
di
HO, HOOH + HO
(a) Is aspartame chiral? If so, how many stereoiso-
mers are possible for it?
(c)
(b) Name each functional group in aspartame. H
Estimate the net charge on an aspartame
molecule in aqueous solution at pH 7.0.
Would you expect aspartame to be soluble in
water? Explain. (Chapter 5)
CUDIET
(e) Draw structural formulas for the products of com-
plete hydrolysis of aspartame in aqueous HCI.
Show each product as it would be ionized in this
1
Ques
gune
solution.
OCH;100.HO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning