1-The answer rounded to the correct number of significant figures after Performing the following calculations (2.430 + 1.871×10 ) x 4.672 is ... 2- The melting point of gold is 1948 °F, this melting point in degrees of °C is 3- A compound containing only chlorine and oxygen is 53.0% oxygen by mass. The empirical formula of the compound is .. .
1-The answer rounded to the correct number of significant figures after Performing the following calculations (2.430 + 1.871×10 ) x 4.672 is ... 2- The melting point of gold is 1948 °F, this melting point in degrees of °C is 3- A compound containing only chlorine and oxygen is 53.0% oxygen by mass. The empirical formula of the compound is .. .
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter1: Essential Ideas
Section: Chapter Questions
Problem 77E: Make the conversion indicated in each of the following: the length of a soccer field, 120 m (three...
Related questions
Question
please fast
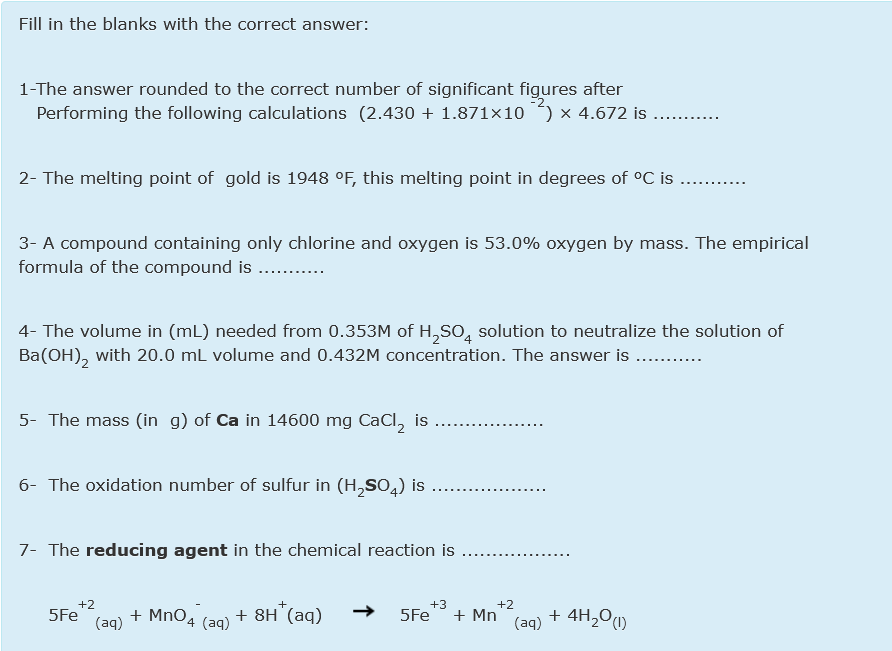
Transcribed Image Text:Fill in the blanks with the correct answer:
1-The answer rounded to the correct number of significant figures after
Performing the following calculations (2.430 + 1.871×10
x 4.672 is
2- The melting point of gold is 1948 °F, this melting point in degrees of °C is
3- A compound containing only chlorine and oxygen is 53.0% oxygen by mass. The empirical
formula of the compound is .. .
4- The volume in (mL) needed from 0.353M of H,SO, solution to neutralize the solution of
Ba(OH), with 20.0 mL volume and 0.432M concentration. The answer is ...
5- The mass (in g) of Ca in 14600 mg CaCl, is
6- The oxidation number of sulfur in (H,SO,) is ..
7- The reducing agent in the chemical reaction is
+2
5Fe
+3
+2
+ MnO4
+ 8H (aq)
+ Mn
+ 4H,O1)
5Fe
(aq)
4 (aq)
(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning