1) What are the coefficients of the balance equation? 2) What is the total number of electrons lost/gained? 3) What is the substance that undergo oxidation reaction? reduction reaction? 4) Which of the reactants is the oxidizing agent? reducing agent? 5) Which substance was oxidized? reduced? 6) Which compound is the product of oxidation? product of reduction?
1) What are the coefficients of the balance equation? 2) What is the total number of electrons lost/gained? 3) What is the substance that undergo oxidation reaction? reduction reaction? 4) Which of the reactants is the oxidizing agent? reducing agent? 5) Which substance was oxidized? reduced? 6) Which compound is the product of oxidation? product of reduction?
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter10: Acids, Bases, And Salts
Section: Chapter Questions
Problem 10.71EP
Related questions
Question
1) What are the coefficients of the balance equation?
2) What is the total number of electrons lost/gained?
3) What is the substance that undergo oxidation reaction? reduction reaction?
4) Which of the reactants is the oxidizing agent? reducing agent?
5) Which substance was oxidized? reduced?
6) Which compound is the product of oxidation? product of reduction?
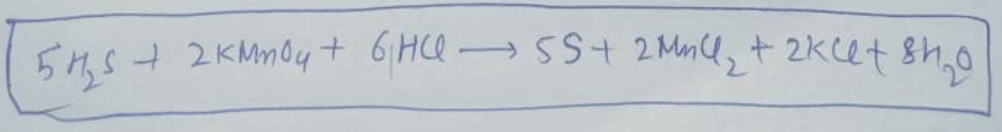
Transcribed Image Text:54,5+ 2KMm04t 6 HCQ SS+ 2 Mml, + 2kcet 84.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
