1. 2. Silica 3. the column frits. 4. Surface metal ions act as multiple polar groups. is less stable than particles can cause peak broadening while at high pH. (silica, zirconia) has a larger surface area than particle type can clog agents to retain analyte molecules with
1. 2. Silica 3. the column frits. 4. Surface metal ions act as multiple polar groups. is less stable than particles can cause peak broadening while at high pH. (silica, zirconia) has a larger surface area than particle type can clog agents to retain analyte molecules with
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.28QAP
Related questions
Question
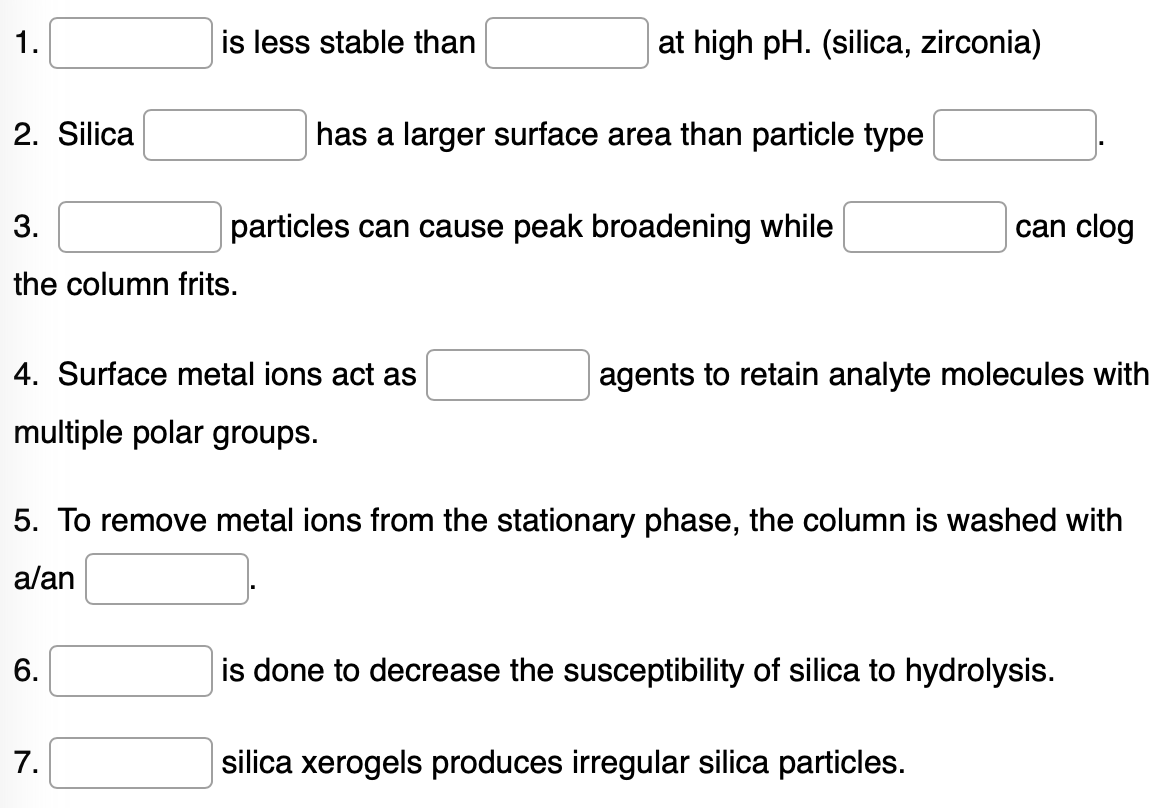
Transcribed Image Text:1.
2. Silica
3.
can clog
the column frits.
4. Surface metal ions act as
agents to retain analyte molecules with
multiple polar groups.
5. To remove metal ions from the stationary phase, the column is washed with
a/an
6.
is done to decrease the susceptibility of silica to hydrolysis.
7.
silica xerogels produces irregular silica particles.
is less stable than
particles can cause peak broadening while
at high pH. (silica, zirconia)
has a larger surface area than particle type
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning