1. A 300 mole mixture that is 30.0 mol% acetic acid at 0.4 bar is compressed isothermally. At what pressure will the first vapor bubble form from this mixture? 2. The mixture in (a) is further compressed to 1.2 bar. What is the amount and composition of the liquid and/or vapor phases formed from the mixture at this pressure? 3. At what pressure will the last drop of liquid from the mixture vaporize? Note: Use a tolerance value of 1 x 10-² in calculations. Do it manually.
1. A 300 mole mixture that is 30.0 mol% acetic acid at 0.4 bar is compressed isothermally. At what pressure will the first vapor bubble form from this mixture? 2. The mixture in (a) is further compressed to 1.2 bar. What is the amount and composition of the liquid and/or vapor phases formed from the mixture at this pressure? 3. At what pressure will the last drop of liquid from the mixture vaporize? Note: Use a tolerance value of 1 x 10-² in calculations. Do it manually.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
please answer all, show all the necessary solution. thank you.
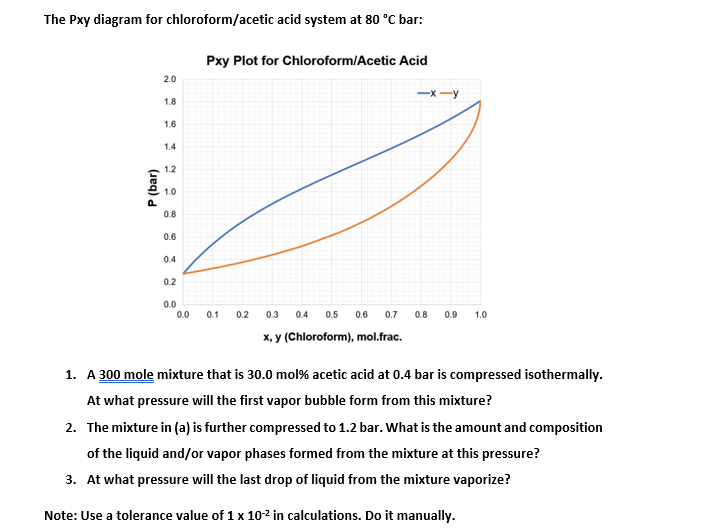
Transcribed Image Text:The Pxy diagram for chloroform/acetic acid system at 80 °C bar:
P (bar)
2.0
1.8
1.6
1.4
1.2
1.0
0.8
0.6
0.4
0.2
0.0
0.0
Pxy Plot for Chloroform/Acetic Acid
0.1 0.2
-x-y
0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
x, y (Chloroform), mol.frac.
1. A 300 mole mixture that is 30.0 mol% acetic acid at 0.4 bar is compressed isothermally.
At what pressure will the first vapor bubble form from this mixture?
2. The mixture in (a) is further compressed to 1.2 bar. What is the amount and composition
of the liquid and/or vapor phases formed from the mixture at this pressure?
3. At what pressure will the last drop of liquid from the mixture vaporize?
Note: Use a tolerance value of 1 x 10-² in calculations. Do it manually.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The