1. A Rolaids tablet contains calcium carbonate that neutralizes stomach acid. If 44.55 mL of 0.448 M hydrochloric acid is required to neutralize one tablet, how many milligrams of calcium carbonate are in a Rolaids tablet? CaCO, (s) + 2HCl(aq) → CaCl₂ (aq) + H₂O (1) + CO₂(g)
1. A Rolaids tablet contains calcium carbonate that neutralizes stomach acid. If 44.55 mL of 0.448 M hydrochloric acid is required to neutralize one tablet, how many milligrams of calcium carbonate are in a Rolaids tablet? CaCO, (s) + 2HCl(aq) → CaCl₂ (aq) + H₂O (1) + CO₂(g)
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.12E
Related questions
Question
100%

Transcribed Image Text:Concentration of Acetic Acid in a Vinegar Solution.
Average molarity of
NaOH
Volume HC₂H30₂
sample
Final buret reading
Initial buret reading
Volume NaOH
(Show all units and all
calculations)
Molarity of HC₂H30₂
(unrounded)
Average molarity
of HC₂H302
(unrounded)
Deviations
Average deviation
Best estimate for
molarity of
HC₂H302
Average mass percent
HC₂H302
46.4mL
Oml
Ran out of Base
↓ ↓
↓ ↓
10.014
HOA
je beart
giov
isults weari?!
ferottelucuny
Cambia)
10
Kousad
Question
1. A Rolaids tablet contains calcium carbonate that neutralizes stomach acid. If 44.55 mL of 0.448
M hydrochloric acid is required to neutralize one tablet, how many milligrams of calcium
carbonate are in a Rolaids tablet?
CaCO, (s) + 2HCl(aq) → CaCl₂ (aq) + H₂O (1) + CO₂ (g)
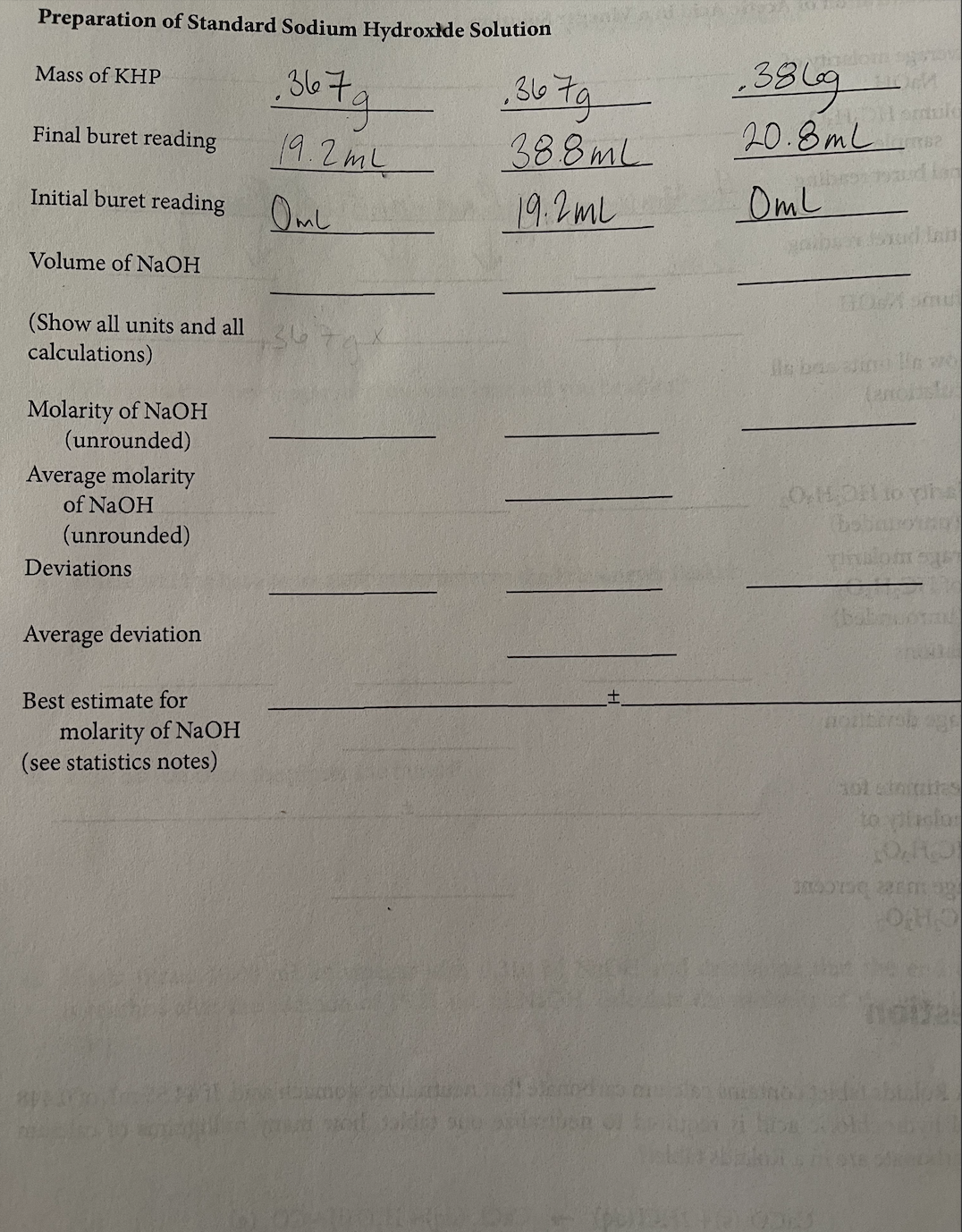
Transcribed Image Text:Preparation of Standard Sodium Hydroxide Solution
1.367g
Mass of KHP
Final buret reading
Initial buret reading
Volume of NaOH
(Show all units and all
calculations)
Molarity of NaOH
(unrounded)
Average molarity
of NaOH
(unrounded)
Deviations
Average deviation
Best estimate for
molarity of NaOH
(see statistics notes)
19.2mL
OML
.367g
38.8mL
19.2mL
Witbankstumok
nd! Startindi
un sot jaldin su legion
ondom ogszov
2100M
وما38.
20.8ml 2
Oml
is bas ating I'm wo
(anoista
Vakrabzon
Soud lan
0.1.011 to ha
ensina
vinulom r
TAN
nozibero 238
sot clarantes
to placius
O,HO
300075 200 g
Skech
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning