1. A solution of HCIO, was standardized by dissolving 0.3745 g of primary standard grade HgO in the solution of KBr. The liberated OH required 37.79 mL of the acid to be neutralized. Calculate the molarity of HCIO.. HgOs) + 4Br + H,0 > HgBr. + 20H
Q: Why use basic buffer solution in an experiment for hard water analysis?
A: Buffer solution is solution which resist the change in pH of solution.
Q: Select the metal that can protect iron from corrosion. EºFe = -0.44 V chromium, Cr, E° = -0.74 V…
A: metal which is more reactive than Iron can protect it from corrosion o
Q: What is Δ G for the reaction below at 298K with a partial pressure of nitrogen dioxide of 4.0 atm.…
A:
Q: The pH of a 0.24M solution of pentanoic acid (HC, H,0,) is measured to be 2.73. Calculate the acid…
A: In this question we have to tell the pH of the solution.
Q: N. Br2/FeBr3 CO2CH3 So,/H2SO4 HO. AICI3 NO2 HNO3/H2SO4 CN
A: Reaction 1 : EAS Bromine is electrophile. Reaction 2 : EAS SO3 is electrophile. Reaction 3 : Fridel…
Q: 3. A solution containing 0.150 g of lysozyme in 210. ML of solution has an osmotic pressure of 2.12…
A:
Q: For the reaction 20,H(g) + 70,(g) 4CO, (g) + 6H,0(g) (a) Predict the enthalpy of reaction from the…
A: The reaction given is,
Q: Discuss other medical applications on the use of EDTA to treat poisonings by radioactive materials…
A:
Q: Ethyl acetate (C4H8O2, mm=88.10 g/mol) is a colorless liquid often used in nail polish removers,…
A: Note: As per the guidelines, solution of the first question has been made. For the expert solution…
Q: What is the maximum wavelength capable of breaking an oxygen - oxygen bond in ozone? Both of the…
A: The bond enthalpy of ozone is 362.8 KJ/molBoth of the bonds in ozone are equivalent having a bond…
Q: what are the vibrational bands of ASA
A: A question based on IR spectroscopy that is to be accomplished.
Q: A certain system, made up of distinguishable particles, has three nondegenarate energy levels that…
A:
Q: Chapter 11: Nucleic Acids Select and draw one of the five bases (adenine, cytosine, guanine,…
A: To draw one of the bases Convert it to nucleotide Number the carbons on the sugar
Q: OR OR OH H20 OH но OR OR
A:
Q: 1) The attached picture shows the structure of aluminum axide the column chromatography stationary…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Complete the following for each of the following structures below. Determine whether it is an…
A:
Q: Use the observations about each chemical reaction in the table below to decide the sign (positive or…
A: The observations given are,
Q: The Ka value for chlorous acid (HCIO₂) is 1.1 × 10-2, while the Ka for nitrous acid (HNO₂) is 4.6 x…
A: Give that, The Ka of the chlorous acid (HClO2) is 1.1×10-2. The Ka of the nitrous acid (HNO2) is…
Q: Given the following information, calculate the reaction velocity at saturating substrate…
A: Given that, for an enzyme kinetics reaction [S] = 100 mM = 0.1 M, k1 = 10 s-1, k2 = 3000 s-1, k-1 =…
Q: If 100 g of Cu at 80°C is dropped into 50 g of water at 20°C, what will be the final T of the water…
A:
Q: 1) NAOH, heat Br d) 2) MCPBA Br 1) KOCCH,), heat 2) H,0
A:
Q: Question 3:, following transformations. Make sure to include any proton transfer that takes place as…
A: below full mechanism step by step is show
Q: The heat of fusion AH, of propane (C3Hg) is 3.5 kJ/mol. Calculate the change in entropy AS when 2.1…
A: Here we are required to find the entropy when 2.1gm of propane freeze
Q: 1. You do not have any acetic acid or acetic anhydride. You use instead acetyl chloride (molar mass…
A: We first determine the limiting reagent by calculating the moles of reactant. The reactant which is…
Q: What functional group is highlighted in this section of an unknown drug? "The highlighted functional…
A: The correct assignment of the nomenclature to the given compound depends on the position of the Br…
Q: A baseball pitcher's fastballs have been clocked at about 94 mph (1 mile = 1609 m). (a) Calculate…
A: Given data :
Q: Calculate equilibrium constant for the galvanic cell composed of a bromate/bromide half-cell and a…
A:
Q: Please show complete mechanism with all steps with naming OH OH H2, Pd/C 'N' HO МеОн, EtOH 23 °C,…
A: Note : Benzyl group can be deprotected using hydrogenation conditions. Cbz group also can be…
Q: acid has a pH of 2.50 (show work for calculations). Determine the [H3O+] concentration. Determine…
A: Given, pH = 2.50 pH = -log([H3O+]) 2.50 = -log([H3O+)] [H3O+] = 0.00316 M [OH-] = Kw/[H+] =…
Q: II. lodometry II. Permanganate IV. Dichromate
A: Indicators can be defined as the substances that change colour when they are added to basic or…
Q: Angiotensin II is a polypeptide that regulates blood pressure. 2nd attempt Enter the amino acids, in…
A:
Q: A reaction mechanism is show below A +B → C step-1 step-2 step-3 step-4 C+D→ E C+C→ C2 E → D+F What…
A: Write balanced equation for the reaction ?
Q: What is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to…
A: In this question we have to tell the PH of the buffer solution after adding 0.04 mol of HCl.
Q: Br. CH,C, (ooverte) os 1.0, 2. (CH,),S KMno, (hot.conc)
A: These reactions mainly involved highly reactive olefinic bond towards several potential reagents.
Q: What is true about this reaction? A CN is the reducing agent B CN is the oxidizing agent C An oxygen…
A:
Q: Ag wire used to measure the concentration of CI ion is an example of a what type of electrode? O…
A:
Q: Which of the following statements is correct if any of these compounds underwent a substitution…
A: C
Q: Question 3:, following transformations. Make sure to include any proton transfer that takes place as…
A: below mechanism is given
Q: (Q69) Using the tabulated values from the text (located in Appendix G, beginning at p. 1211)…
A: Calculate gibbs free energy of the given reaction---
Q: 1) Nal Br a) 2) NaCN, acetone I) NAOC(CH,), heat b) Br 2) BH, THF; 3) H,O, NaOH(aq) 1) HCI c) 2)…
A: Since you have posted a question with multiple sub-parts, we will solve the first three subparts for…
Q: 4. Draw and list all the symmetry elements, and determine the point groups for the following…
A:
Q: 1. Calculate the AS of the given reaction. H2 (9) + H2 (9) H2O (g. O2 (g) 305.0 O2 (9) → H2O ()…
A: Entropy can be defined as the degree of disorder in system or degree of randomness in system.
Q: Is the production of uric acid wasteful?
A: (1) Yes, Uricothelic organisms tend to excrete uric acid waste in the form of a white paste or…
Q: Ethyl acetate (C4H3O2, mm388.10 g/mol) is a colorless liquid often used in nail polish removers,…
A: Since you have asked multiple question, we will solve the first question for you.If you want any…
Q: 3500 3000 2500 Wavenumber (cm-1) 2000 1500 1000 Identify an unknown alcohol (1-butanol, isoamyl…
A: Infrared spectroscopy is the study of interaction of infrared light with matter, which can be used…
Q: Calculate Δ Go for a redox reaction where two electrons are transferred in the balanced equation and…
A: Given, Standard cell potential = E0 = -0.59 V Number of electrons transferred = n = 2 Value of…
Q: Which of the following statements is not true?
A:
Q: determine what would happen to the position of the equilibrium when the following changes are made…
A: Equilibrium can be defined as the state of reaction in which rate of forward reaction become equal…
Q: 30 215N. Use the atomic masses on the table below to calculate Am for the change 14 Si u Which is…
A: The reaction given is, =>
Q: What are the products A and B in the following Michael Addition? OEI NaOEt EIOH 1. Name or describe…
A: Activated methylene group attacks on β position of eneone. This is called Michael addition.
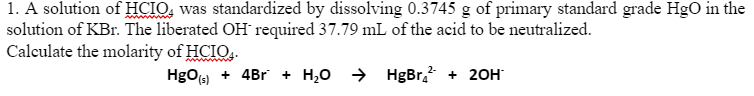
Step by step
Solved in 2 steps with 2 images

- calculate the mass of Sn when 50 mL of a sample containing Sn2+ is titrated with 42.00 mL of 0.0150M MnO4- to reach endpointThe level of dissolved oxygen in a water sample can be determined by the Winkler method. In a typical analysis, a 100.0-mL sample is made basic, and treated with a solution of MnSO4, resulting in the formation of MnO2. An excess of KI is added, and the solution is acidified, resulting in the formation of Mn2+ and I2. The liberated I2 is titrated with a solution of 0.00870 M Na2S2O3, requiring 8.90 mL to reach the starch indicator end point. Calculate the concentration of dissolved oxygen as parts per million of O2.3 L contaminated air 50 mL 0.0116 M in an air pollution analysisCarbon dioxide (CO2) BaCO3 is passed through Ba (OH) 2 solution.is precipitated as. Excess of base, next to phenol phthalate (f.f.) indicatorIt is titrated with 23.6 mL of 0.0108 M HCl. CO2 in this air sampleCalculate its concentration in ppm. (Density of CO2Take it as 1.98 g / L. C = 12, O = 16 g / mol).
- A 0.3285 g sample containing chloride and inert material is titrated with 0.1012 M AgNO3, requiring 34.99 mL to reach the Ag,Cro. end point. Titration of a blank sample consumes 0.32 mL of the AgNO, titrant. Determine the % w/w chloride in the sample.0.0585 g Na2C2O4 10 mL to adjust KMnO4 solution prepared in 0.1 M'pure water, 2 M H2SO4 added, heating process and 8.4 mL titrant as a result of titration it's spin out. Calculate the actual concentration of potassium permanganate accordingly1. 0.646 g of sample containing BaCl2.2H2O (244.26 g/mol) was dissolved and enoughpotassium chromate was added. After filtering, the precipitate was dissolved in acid andenough KI was added and titrated with thiosulfate. Since 48.7 mL of 0.137 M thiosulfateis used for this, what is the percentage of BaCl2.2H2O in the sample?K2Cr2O7 + 7H2SO4 + 6KI 4K2SO4 + Cr2(SO4)3 + 7H2O + 3I2
- The arsenic in a 1.22-g sample of a pesticide was converted toAsO43- by suitable chemical treatment. It was then titratedusing Ag+ to form Ag3AsO4 as a precipitate. (a) What is theoxidation state of As in AsO43-? (b) Name Ag3AsO4 by analogyto the corresponding compound containing phosphorusin place of arsenic. (c) If it took 25.0 mL of 0.102 M Ag+to reach the equivalence point in this titration, what is themass percentage of arsenic in the pesticide?A 0.4671 g sample containg NaHCO₃(Mwt=84.01mg/mmol) was dissolved and titrated with 0.1067 M HCl requiring 40.72 ml, find the percentage of NaHCO₃, in the sampleWhat weight of sample should be taken for analysis sos that the volume of 0.1074N NaOH used for titration equals the percentage of potassium acid phthalate (KHC8H4O4) in the sample (Answer 2,193 g)
- 5) 3 L contaminated air 50 mL 0.0116 M in an air pollution analysis Carbon dioxide (CO2) BaCO3 is passed through Ba (OH) 2 solution. as precipitated. Excess of base, next to phenol phthalate (f.f.) indicator It is titrated with 23.6 mL of 0.0108 M HCl. CO2 in this air sample Calculate its concentration in ppm. (Density of CO2 Take it as 1.98 g / L. C = 12, O = 16 g / mol).16.The titration of an aliquot of 6 mL of acetic acid of commercial use, with NaOH 0.1 , was made by titration, so that to reach the equivalence point 40 ml of NaOH were consumed.Determine the percentage concentration (m/m) of the acetic acid, if its density is 1.05 g/ml. HC2H3O2 acetic acid0.5366 g of an KHP sample of unknown purity was massed. The sample was dissolved in approximately 100 mL of distilled, degassed water and indicator was added. The end point was reached after 21.35 mL of 0.09854 M NaOH solution was titrated into the solution. What is the percentage of KHP in the original sample?

