1. Amixture of ethylene and nitrogen is fed to a reactor in which some of the ethylene is dimerized to butene. 100 mol/s REACTOR 0.60 mol C,H/mol 0.40 mol N/mol i, mol CH/s n, mol CH/s iz mol Ny/s 2C,H, - How many independent molecular species are involved in the process? How many independent atomic species are involved? Prove the latter claim by writing balances on C, H, and N.
1. Amixture of ethylene and nitrogen is fed to a reactor in which some of the ethylene is dimerized to butene. 100 mol/s REACTOR 0.60 mol C,H/mol 0.40 mol N/mol i, mol CH/s n, mol CH/s iz mol Ny/s 2C,H, - How many independent molecular species are involved in the process? How many independent atomic species are involved? Prove the latter claim by writing balances on C, H, and N.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 9RQ: What characterizes an electrolytic cell? What is an ampere? When the current applied to an...
Related questions
Question
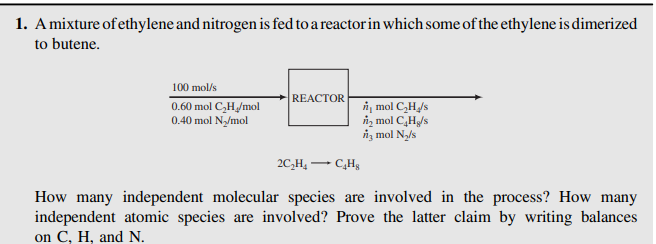
Transcribed Image Text:1. Amixture of ethylene and nitrogen is fed to a reactor in which some of the ethylene is dimerized
to butene.
100 mol/s
REACTOR
0.60 mol C,H/mol
0.40 mol N/mol
i, mol CH/s
n, mol CH/s
iz mol Ny/s
2C,H, -
How many independent molecular species are involved in the process? How many
independent atomic species are involved? Prove the latter claim by writing balances
on C, H, and N.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
