1. An acid-base titration is performed in which 26.05 mL of 0.1000M H₂SO4 solution was needed to reach the endpoint of a 25mL NaOH solution with unknown concentration. The chemical reaction involved in this titration is as follows: H₂SO4 (aq) + 2NaOH(aq) → H₂O + Na₂SO4 (aq) Calculate for the molarity of the NaOH solution.
1. An acid-base titration is performed in which 26.05 mL of 0.1000M H₂SO4 solution was needed to reach the endpoint of a 25mL NaOH solution with unknown concentration. The chemical reaction involved in this titration is as follows: H₂SO4 (aq) + 2NaOH(aq) → H₂O + Na₂SO4 (aq) Calculate for the molarity of the NaOH solution.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 11E: The Handbook of Chemistry and Physics (http://openstaxcollege.org/l/16Handbook) gives solubilities...
Related questions
Question
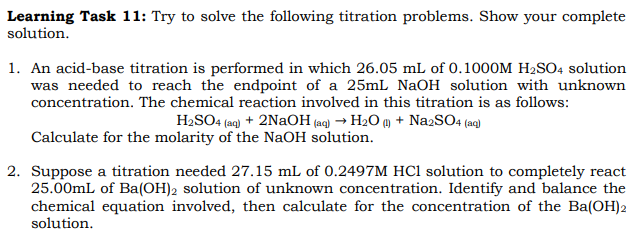
Transcribed Image Text:Learning Task 11: Try to solve the following titration problems. Show your complete
solution.
1. An acid-base titration is performed in which 26.05 mL of 0.1000M H₂SO4 solution
was needed to reach the endpoint of a 25mL NaOH solution with unknown
concentration. The chemical reaction involved in this titration is as follows:
H₂SO4 (aq) + 2NaOH(aq) → H₂O (1) + Na2SO4 (aq)
Calculate for the molarity of the NaOH solution.
2. Suppose a titration needed 27.15 mL of 0.2497M HCl solution to completely react
25.00mL of Ba(OH)2 solution of unknown concentration. Identify and balance the
chemical equation involved, then calculate for the concentration of the Ba(OH)2
solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax