Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter4: Stoichiometry Of Chemical Reactions
Section: Chapter Questions
Problem 86E: Sodium bicarbonate (baking soda), NaHCO3, can be purified by dissolving it in hot water (60 C),...
Related questions
Question
Transcribed Image Text:Document (35)
Home
Insert
Draw
Layout
Review
View
Calibri Regular (B
11
I
A..
E E E
A
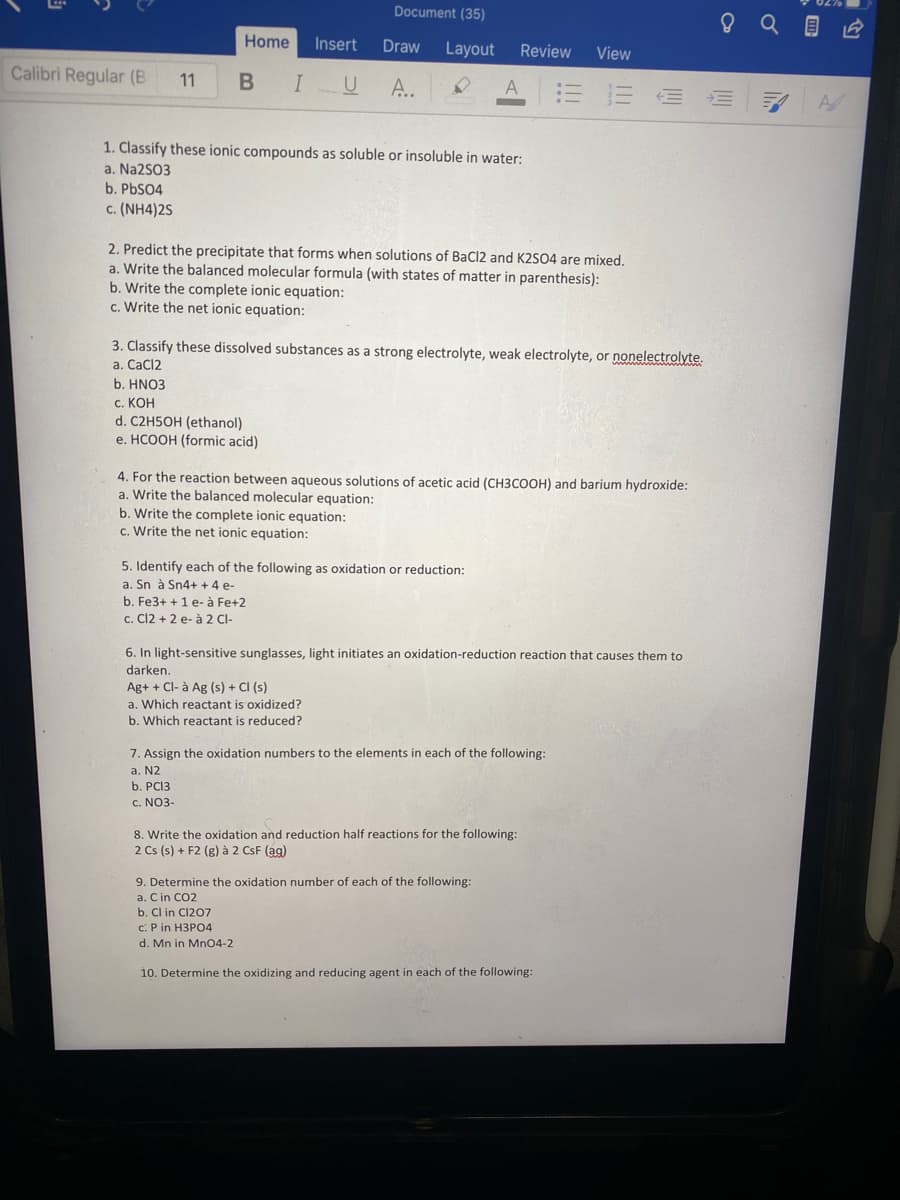
1. Classify these ionic compounds as soluble or insoluble in water:
a. Na2S03
b. PbS04
c. (NH4)2S
2. Predict the precipitate that forms when solutions of BaCl2 and K2SO4 are mixed.
a. Write the balanced molecular formula (with states of matter in parenthesis):
b. Write the complete ionic equation:
c. Write the net ionic equation:
3. Classify these dissolved substances as a strong electrolyte, weak electrolyte, or nonelectrolyte.
a. CaCl2
b. HNO3
с. КОН
d. C2H5OH (ethanol)
e. HCOOH (formic acid)
4. For the reaction between aqueous solutions of acetic acid (CH3COOH) and barium hydroxide:
a. Write the balanced molecular equation:
b. Write the complete ionic equation:
c. Write the net ionic equation:
5. Identify each of the following as oxidation or reduction:
a. Sn à Sn4+ + 4 e-
b. Fe3+ + 1 e- à Fe+2
c. Cl2 + 2 e- à 2 Cl-
6. In light-sensitive sunglasses, light initiates an oxidation-reduction reaction that causes them to
darken.
Ag+ + Cl- à Ag (s) + CI (s)
a. Which reactant is oxidized?
b. Which reactant is reduced?
7. Assign the oxidation numbers to the elements in each of the following:
a. N2
b. PCI3
c. NO3-
8. Write the oxidation and reduction half reactions for the following:
2 Cs (s) + F2 (g) à 2 CsF (ag)
9. Determine the oxidation number of each of the following:
a. Cin CO2
b. Cl in Cl207
c: P in H3PO4
d. Mn in Mn04-2
10. Determine the oxidizing and reducing agent in each of the following:
!!

Transcribed Image Text:Calibri Regular (B
BIUA..
11
8. Write the oxidation and reduction half reactions for the following:
2 Cs (s) + F2 (g) à 2 CsF (ag)
9. Determine the oxidation number of each of the following:
a. Cin CO2
b. Cl in Cl207
C. P in H3PO4
d. Mn in Mn04-2
10. Determine the oxidizing and reducing agent in each of the following:
a. Zn (s) + 2 HBr (ag) à ZnBr2 (ạag) + H2 (g)
b. Mn (s) + Pb(NO3)2 (ag) à Pb (s) + Mn(NO3)2 (ag)
11. For the following reaction:
2 Na + 2 H20 à 2 NaOH + H2
a. Write the oxidation number for each element in the reaction
b. Write the reduction and oxidation half reactions
c. Identify the reducing and oxidizing agents
12. How many liters of H2 gas are produced when 6.25 g of Zn react with 20.0 mL of a 1.50 M HCI
solution?
Zn (s) + 2 HCI (ag) à ZnCl2 (ag) + H2 (g)
13. How many grams of 02 react if 1280 kJ was released in the following reaction?
CH4 + 2 02 à CO2 + 2 H2O DH = -890 kJ
14. Aluminum is used to make kitchen utensils. What is the mass of an aluminum spatula if 3.25 kJ of
heat raises its temperature from 20.0 °C to 45.0 °C. SHAI = 0. 897 J/g °C?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning