Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 57P: An ideal gas expands isothermally along AB and does 700 J of work (see below). (a) How much heat...
Related questions
Question
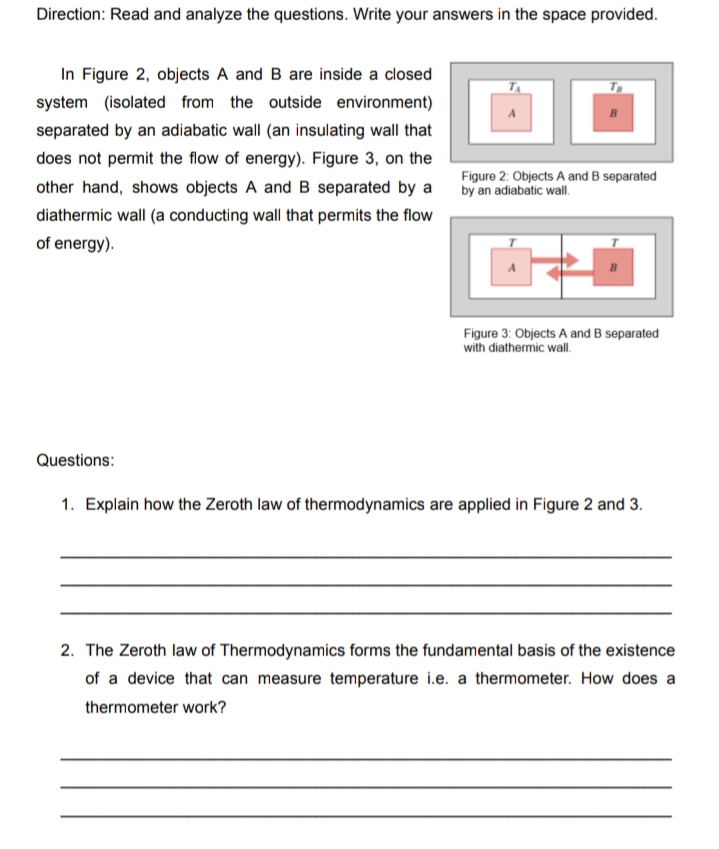
Transcribed Image Text:Direction: Read and analyze the questions. Write your answers in the space provided.
In Figure 2, objects A and B are inside a closed
system (isolated from the outside environment)
separated by an adiabatic wall (an insulating wall that
does not permit the flow of energy). Figure 3, on the
other hand, shows objects A and B separated by a
Figure 2: Objects A and B separated
by an adiabatic wall.
diathermic wall (a conducting wall that permits the flow
of energy).
Figure 3: Objects A and B separated
with diathermic wall.
Questions:
1. Explain how the Zeroth law of thermodynamics are applied in Figure 2 and 3.
2. The Zeroth law of Thermodynamics forms the fundamental basis of the existence
of a device that can measure temperature i.e. a thermometer. How does a
thermometer work?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning