1. First, 0.9144 grams of the material was placed into an evacuated 0.500 L container. The temperature was raised to 600.0 K, and the resulting pressure was 1.00 atm. Calculate the molar mass of the unknown material?
1. First, 0.9144 grams of the material was placed into an evacuated 0.500 L container. The temperature was raised to 600.0 K, and the resulting pressure was 1.00 atm. Calculate the molar mass of the unknown material?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 112SCQ
Related questions
Question
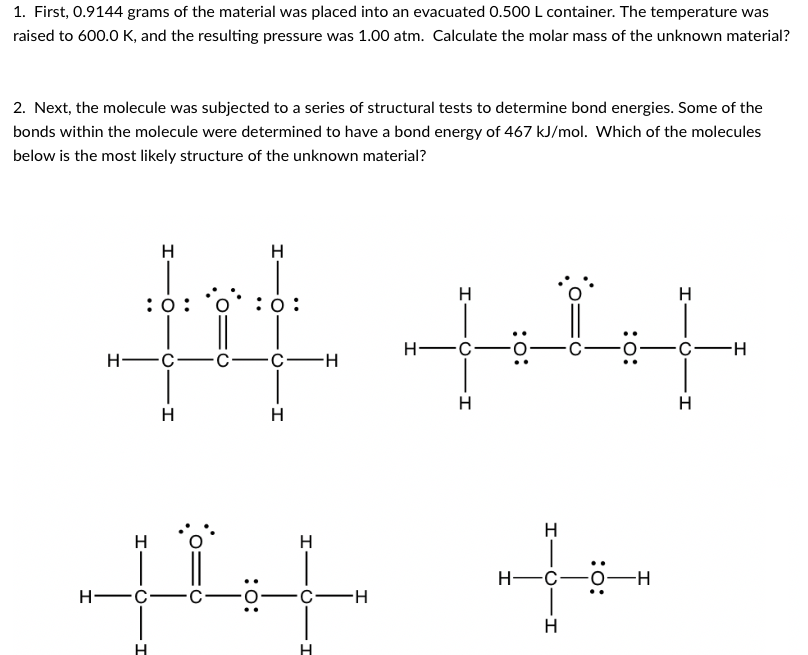
Transcribed Image Text:1. First, 0.9144 grams of the material was placed into an evacuated 0.500 L container. The temperature was
raised to 600.0 K, and the resulting pressure was 1.00 atm. Calculate the molar mass of the unknown material?
2. Next, the molecule was subjected to a series of structural tests to determine bond energies. Some of the
bonds within the molecule were determined to have a bond energy of 467 kJ/mol. Which of the molecules
below is the most likely structure of the unknown material?
H
H
H
H
:0:
:0:
H FC
H -C-
С —с—н
H
H
H
H
H
H
Н—с—о—н
Н—с.
H
Expert Solution
Step 1
“Since you have asked multiple questions, we will solve the first question for you. If you want any specific question to be solved, then please specify the question number or post only that question.”
The quantities which come across during the scientific studies are named as physical quantities. The standard of reference used to measure any physical quantity is termed as unit. The density of a substance is calculated by dividing its mass by its volume. It is an intensive property.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
