1. For the following reaction 5 CO(g) + 1205(s) → 12(g) + 5 CO2(g) AHO=-1175 kJ for each change listed, predict the equilibrium shift and the effect on the indicated quantity.
1. For the following reaction 5 CO(g) + 1205(s) → 12(g) + 5 CO2(g) AHO=-1175 kJ for each change listed, predict the equilibrium shift and the effect on the indicated quantity.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter13: Chemical Equilibrium
Section: Chapter Questions
Problem 117CP: A mixture of N2, H2, and NH3 is at equilibrium [according to the equationN2(g)+3H2(g)2NH3(g)] as...
Related questions
Question
100%
Provide clear explanation and show work. Clear writing or typed please
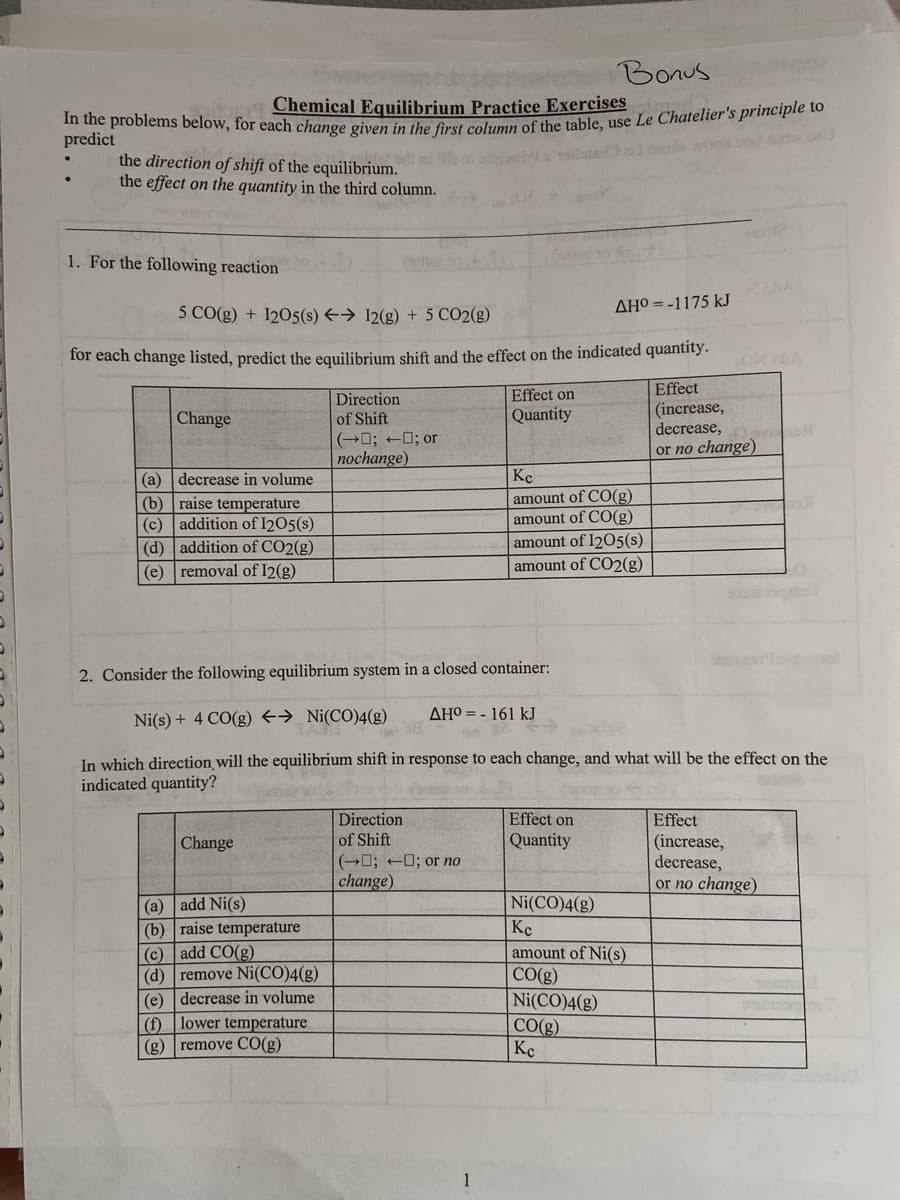
Transcribed Image Text:Bonus
Chemical Equilibrium Practice Exercises
In the problems below, for each change given in the first column of the table, use Le Chatelier's principle to
predict
the direction of shift of the equilibrium.
the effect on the quantity in the third column.
1. For the following reaction
Change
5 CO(g) + 1205(s) → 12(g) + 5 CO2(g)
for each change listed, predict the equilibrium shift and the effect on the indicated quantity.
Effect
(increase,
decrease,
or no change)
(a) decrease in volume
(b) raise temperature
(c)
addition of 1205(s)
(d)
addition of CO2(g)
(e) removal of I2(g)
Change
Direction
of Shift
(a) add Ni(s)
(b) raise temperature
(c) add CO(g)
(d) remove Ni(CO)4(g)
(e) decrease in volume
(f) lower temperature
(g) remove CO(g)
(-; -; or
nochange)
Direction
of Shift
Effect on
Quantity
(0; 0; or no
change)
1
al mode wil
2. Consider the following equilibrium system in a closed container:
Ni(s) + 4 CO(g) → Ni(CO)4(g)
In which direction will the equilibrium shift in response to each change, and what will be the effect on the
indicated quantity?
AHO = - 161 kJ
AHO=-1175 kJ
Kc
amount of CO(g)
amount of CO(g)
amount of 1205(s)
amount of CO2(g)
Effect on
Quantity
Dovano
Ni(CO)4(g)
Кс
amount of Ni(s)
CO(g)
Ni(CO)4(g)
CO(g)
Кс
Swis
Effect
(increase,
decrease,
or no change)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning