1. How many protons (P), neutrons (N) and electrons (e) are present in 22286Rn+²: a. 222 p 136 n 86 e b. 86 p 133 n 84 e c. 86 p 136 n 84 e d. 84 p 133 n 84 e.
1. How many protons (P), neutrons (N) and electrons (e) are present in 22286Rn+²: a. 222 p 136 n 86 e b. 86 p 133 n 84 e c. 86 p 136 n 84 e d. 84 p 133 n 84 e.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.103P
Related questions
Question
solve very very fast in 15 min | I need a clear answer by hand, not by keyboard
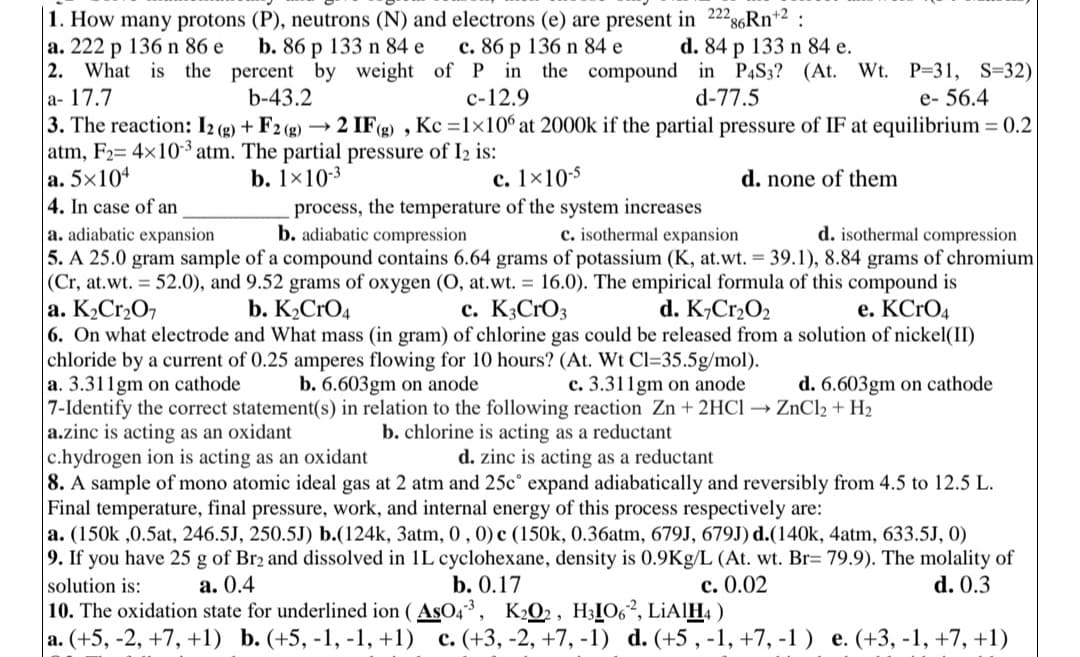
Transcribed Image Text:1. How many protons (P), neutrons (N) and electrons (e) are present in 2228
a. 222 p 136 n 86 e
b. 86 p 133 n 84 e
2. What is the percent by weight of
a-17.7
b-43.2
3. The reaction: 12(g) + F2 (g) -> 2 IF(g), Kc =1×106 at 2000k if the partial pressure of IF at equilibrium = 0.2
atm, F2= 4x10-3 atm. The partial pressure of 1₂ is:
a. 5x104
b. 1x10-3
c. 1x10-5
d. none of them
4. In case of an
c. 86 p 136 n 84 e
286Rn+2:
d. 84 p 133 n 84 e.
P in the compound in P4S3? (At. Wt. P-31, S=32)|
c-12.9
d-77.5
e-56.4
process, the temperature of the system increases
b. adiabatic compression
c. isothermal expansion
a. adiabatic expansion
d. isothermal compression
5. A 25.0 gram sample of a compound contains 6.64 grams of potassium (K, at.wt. = 39.1), 8.84 grams of chromium
(Cr, at.wt. = 52.0), and 9.52 grams of oxygen (O, at.wt. = 16.0). The empirical formula of this compound is
a. K₂Cr₂O7
c. K3CrO3
d. K₂Cr₂O₂
e. KCrO4
b. K₂CRO4
6. On what electrode and What mass (in gram) f chlorine gas could be released from a solution of nickel(II)
chloride by a current of 0.25 amperes flowing for 10 hours? (At. Wt Cl=35.5g/mol).
a. 3.311gm on cathode
b. 6.603gm on anode
c. 3.311gm on anode
d. 6.603gm on cathode
7-Identify the correct statement(s) in relation to the following reaction Zn + 2HCl → ZnCl₂ + H₂
a.zinc is acting as an oxidant
b. chlorine is acting as a reductant
c.hydrogen ion is acting as an oxidant
d. zinc is acting as a reductant
8. A sample of mono atomic ideal gas at 2 atm and 25cº expand adiabatically and reversibly from 4.5 to 12.5 L.
Final temperature, final pressure, work, and internal energy of this process respectively are:
a. (150k ,0.5at, 246.5J, 250.5J) b.(124k, 3atm, 0, 0) c (150k, 0.36atm, 679J, 679J) d.(140k, 4atm, 633.5J, 0)
9. If you have 25 g of Br2 and dissolved in 1L cyclohexane, density is 0.9Kg/L (At. wt. Br= 79.9). The molality of
solution is:
a. 0.4
b. 0.17
d. 0.3
c. 0.02
10. The oxidation state for underlined ion (ASO4³, K2O2, H31O62, LiAlH4)
a. (+5, -2, +7, +1) b. (+5, -1, -1, +1) c. (+3, -2, +7, -1) d. (+5, -1, +7, -1) e. (+3, -1, +7, +1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning