Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter7: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 16CR: The element carbon undergoes many inorganic reactions, as well as being the basis for the field of...
Related questions
Question
answer 1 through 10 blank spaces
Transcribed Image Text:9:47
Chapter 8-Chemical Reactions E...
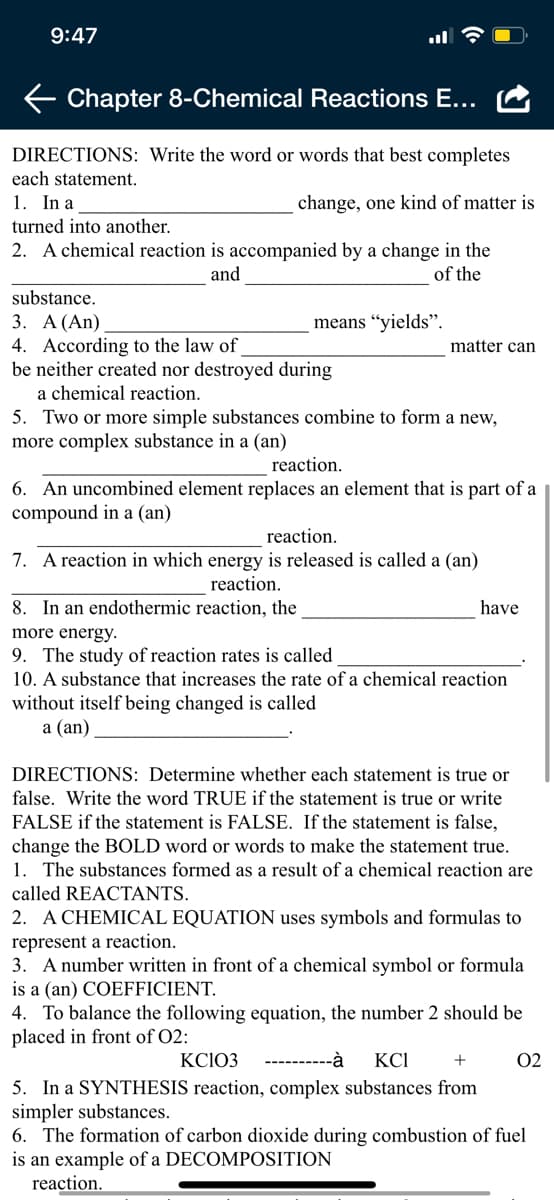
DIRECTIONS: Write the word or words that best completes
each statement.
1. In a
change, one kind of matter is
turned into another.
2. A chemical reaction is accompanied by a change in the
of the
and
substance.
3. А (An)
means “yields".
4. According to the law of
be neither created nor destroyed during
matter can
a chemical reaction.
5. Two or more simple substances combine to form a new,
more complex substance in a (an)
reaction.
6. An uncombined element replaces an element that is part of a
compound in a (an)
reaction.
7. A reaction in which energy is released is called a (an)
reaction.
8. In an endothermic reaction, the
have
more energy.
9. The study of reaction rates is called
10. A substance that increases the rate of a chemical reaction
without itself being changed is called
а (an)
DIRECTIONS: Determine whether each statement is true or
false. Write the word TRUE if the statement is true or write
FALSE if the statement is FALSE. If the statement is false,
change the BOLD word or words to make the statement true.
1. The substances formed as a result of a chemical reaction are
called REACTANTS.
2. A CHEMICAL EQUATION uses symbols and formulas to
represent a reaction.
3. A number written in front of a chemical symbol or formula
is a (an) COEFFICIENT.
4. To balance the following equation, the number 2 should be
placed in front of O2:
KCIO3
----------à
KCI
+
02
5. In a SYNTHESIS reaction, complex substances from
simpler substances.
6. The formation of carbon dioxide during combustion of fuel
is an example of a DECOMPOSITION
reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning