1. Natural gas usually contains a mixture of methane and ethane. Methane (CH4) and ethane (C₂H6) undergo combustion in a furnace. Air, along with another stream of pure O2, are fed to the furnace. The reactor effluent, stream A, has a total molar flow rate, composition, and T and P as shown in the schematic. The reactor effluent is fed to a 1st condenser (C1), in which all of the water (and none of the other chemicals) is condensed and removed. The remaining components are fed to a 2nd condenser (C2). However, the maximum capacity through the condenser is 80 kmol/h; the remainder of the reactor effluent bypasses the condenser. All of the ethane (and none of the other chemicals) in the feed to C2 is condensed (hence the reason that this type of natural gas is given the strange name of "natural gas liquid") and removed. The gas stream leaving C2 is mixed with the bypass stream, to form the stack gas. a. Calculate the volumetric flow rate of stream A, in m³/h b. Calculate the volumetric flow rate of stream A in SCMH c. Calculate the partial pressure of O₂ in stream A. d. Write the chemical reaction(s) that occur in the furnace. e. Completely label the schematic. Note: you do not need to assign variable names when the actual flow rate is obvious. f. Calculate the flow rate of each component in each stream, in kmol/h, and write them on each stream in the schematic. g. Calculate the percent excess oxygen fed to the furnace, considering the total of oxygen fed to the furnace from both the air and pure oxygen streams. h. Calculate the concentration of ethane in the stack gas, in ppm
1. Natural gas usually contains a mixture of methane and ethane. Methane (CH4) and ethane (C₂H6) undergo combustion in a furnace. Air, along with another stream of pure O2, are fed to the furnace. The reactor effluent, stream A, has a total molar flow rate, composition, and T and P as shown in the schematic. The reactor effluent is fed to a 1st condenser (C1), in which all of the water (and none of the other chemicals) is condensed and removed. The remaining components are fed to a 2nd condenser (C2). However, the maximum capacity through the condenser is 80 kmol/h; the remainder of the reactor effluent bypasses the condenser. All of the ethane (and none of the other chemicals) in the feed to C2 is condensed (hence the reason that this type of natural gas is given the strange name of "natural gas liquid") and removed. The gas stream leaving C2 is mixed with the bypass stream, to form the stack gas. a. Calculate the volumetric flow rate of stream A, in m³/h b. Calculate the volumetric flow rate of stream A in SCMH c. Calculate the partial pressure of O₂ in stream A. d. Write the chemical reaction(s) that occur in the furnace. e. Completely label the schematic. Note: you do not need to assign variable names when the actual flow rate is obvious. f. Calculate the flow rate of each component in each stream, in kmol/h, and write them on each stream in the schematic. g. Calculate the percent excess oxygen fed to the furnace, considering the total of oxygen fed to the furnace from both the air and pure oxygen streams. h. Calculate the concentration of ethane in the stack gas, in ppm
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
complete part E only
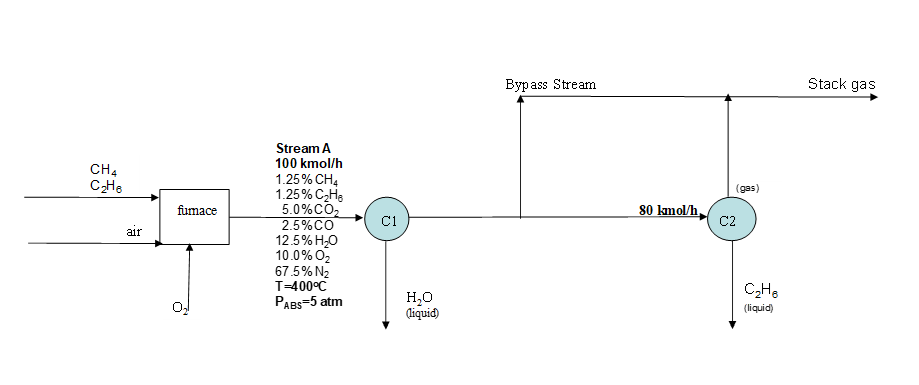
Transcribed Image Text:CH4
C₂H6
air
fumace
0₂
Stream A
100 kmol/h
1.25% CH4
1.25% C₂H₂
5.0% CO₂
2.5%CO
12.5% H₂O
10.0% O₂
67.5% N₂
T=400°C
PABS=5 atm
C1
H₂O
(liquid)
Bypass Stream
80 kmol/h
(gas)
C2
C₂He
(liquid)
Stack gas
Transcribed Image Text:1. Natural gas usually contains a mixture of methane and ethane. Methane (CH4) and ethane (C₂H6) undergo
combustion in a furnace. Air, along with another stream of pure O2, are fed to the furnace. The reactor
effluent, stream A, has a total molar flow rate, composition, and T and P as shown in the schematic. The
reactor effluent is fed to a 1st condenser (C1), in which all of the water (and none of the other chemicals)
is condensed and removed. The remaining components are fed to a 2nd condenser (C2). However, the
maximum capacity through the condenser is 80 kmol/h; the remainder of the reactor effluent bypasses the
condenser. All of the ethane (and none of the other chemicals) in the feed to C2 is condensed (hence the
reason that this type of natural gas is given the strange name of "natural gas liquid") and removed. The gas
stream leaving C2 is mixed with the bypass stream, to form the stack gas.
a. Calculate the volumetric flow rate of stream A, in m³/h
b. Calculate the volumetric flow rate of stream A in SCMH
c. Calculate the partial pressure of O₂ in stream A.
d. Write the chemical reaction(s) that occur in the furnace.
e. Completely label the schematic. Note: you do not need to assign variable names when the actual
flow rate is obvious.
f. Calculate the flow rate of each component in each stream, in kmol/h, and write them on each stream
in the schematic.
g. Calculate the percent excess oxygen fed to the furnace, considering the total of oxygen fed to the
furnace from both the air and pure oxygen streams.
h. Calculate the concentration of ethane in the stack gas, in ppm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The